Abstract
Objective
Recently, numerous studies have yielded inconsistent results regarding the effect of metformin on esophageal cancer risk in type 2 diabetes mellitus patients. The purpose of this study is to systematically assess this effect using meta-analysis.
Methods
We searched clinical studies on metformin and esophageal cancer risk in PubMed, Embase, and the Cochrane Library. After literature screening, a series of meta-analyses were conducted using RevMan 5.3 software. The pooled hazard ratio (HR) and the corresponding 95% confidence interval (CI) were used as the effect size.
Results
Five eligible studies (four cohort studies and one case–control study) were included for our meta-analysis using a random-effect model. The analysis showed that metformin could not reduce esophageal cancer risk in type 2 diabetes mellitus patients (HR 0.88, 95% CI 0.60–1.28, P > 0.05). Subgroup analyses by geographic location showed that metformin significantly reduced esophageal cancer risk in Asian patients with type 2 diabetes mellitus (HR 0.59, 95% CI 0.39–0.91, P = 0.02), without heterogeneity between studies (P = 0.80 and I2 = 0%).
Conclusions
Overall, our systematic review and meta-analysis demonstrate that metformin does not reduce esophageal cancer risk in type 2 diabetes mellitus patients. However, a significant reduction in esophageal cancer risk in Asian populations remains to be clarified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer (EC) is the seventh most common cancer and one of the most malignant cancers in the world, with poor prognosis and a low 5-year survival rate of patients [1]. And it has been confirmed as the sixth leading cause of cancer-related death worldwide [1]. Many studies report increased risks of multiple cancers in T2DM patients [2,3,4]. A meta-analysis by Huang et al. [5] including 17 studies shows that patients with diabetes have a significantly increased risk of EC. As the incidence of EC is expected to increase, EC prevention is of vital importance for reducing the burden of disease for T2DM patients [6].
Metformin is one of the most commonly used drugs for the treatment of T2DM [7]. Accumulating evidences show that metformin may reduce cancer risks including risks of gastric [8], colorectal [9], prostate [10], breast [11] and pancreatic cancers [12] in T2DM patients. Tseng et al. [13] show that metformin may reduce EC incidents in T2DM patients. However, other studies merely show inconsistent results—for example, Becker et al. [14] report that metformin does not associate with an altered risk of EC in T2DM patients. Therefore, we conducted a systematic review and meta-analysis to evaluate the effect of metformin on EC risk in T2DM patients.
Methods
The meta-analysis was conducted based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (PRISMA) 2009 guidelines [15]. Ethical approval was not necessary for this type of analysis.
Search strategy
We searched PubMed, Embase, and the Cochrane Library from their inception to April 2020 using a combination of subject headings and free words, and the reference lists of included studies were manually searched to identify relevant studies. The search strategy was as follows: (“metformin” OR “dimethylbiguanidine” OR “dimethylguanylguanidine” OR “glucophage” OR “metformin hydrochloride” OR “hydrochloride, metformin” OR “metformin HCl” OR “HCl, metformin”) AND (“esophageal cancer” OR “esophageal neoplasm” OR “neoplasm, esophageal” OR “esophagus neoplasm” OR “esophagus neoplasms” OR “neoplasm, esophagus” OR “neoplasms, esophagus” OR “neoplasms, esophageal” OR “cancer of esophagus” OR “cancer of the esophagus” OR “esophagus cancer” OR “cancer, esophagus” OR “cancers, esophagus” OR “esophagus cancers” OR “esophageal cancer” OR “cancer, esophageal” OR “cancers, esophageal” OR “esophageal cancers”).
Eligibility criteria
Studies were selected when they met the following inclusion criteria: (1) cohort or case–control studies or randomized clinical trials published as full-text articles; (2) T2DM patients were enrolled; (3) metformin was used for the treatment of T2DM; (4) the endpoint of interest was the risk of EC; (5) adjusted HRs, odds ratio (OR) or relative risk (RR) and associated 95% confidence intervals (CIs), or sufficient data that could calculate them were reported. The exclusion criteria were defined as follows: (1) narrative or systematic reviews, (2) cell culture or animal experiments, (3) editorials, expert opinions, comments, methodological details, (4) conference abstracts and proceedings.
Data extraction
Two reviewers (Wu and Zhang) independently screened studies for inclusion, extracted data and crosschecked results according to the predefined criteria. Any discrepancy in data extraction was resolved by discussions between the two reviewers or judged by the third reviewer (Zhou). Data were extracted into standard spreadsheets based on the following categories: (1) basic information encompassing the first author’s surname, publication year, country of origin, and study design; (2) clinical characteristics of the enrolled participants: the sample size, age ranges, comparisons, assessments, results, adjusted HRs and their 95% CIs; (3) covariates in the multivariable model; (4) key elements of risk of bias assessments.
Risk of bias assessments
The methodological quality and risk of bias in the included studies were assessed in three dimensions (selection, comparability, outcome or exposure) using the Newcastle–Ottawa scale (NOS), with a maximum of nine stars [16]. Studies presenting ≥ 6 stars were considered high-quality. Risk of bias was assessed independently by two authors (Wu and Zhang). Any discrepancy was resolved by discussions between the two authors or judged by the third reviewer (Zhou).
Statistical analysis
RevMan 5.3 software was used for data analysis. HR was chosen as the pooled estimate. HRs and their 95% CIs were converted to their logarithms and standard errors (SEs). Statistical heterogeneity among studies was evaluated using the I2 statistic and Q test [17, 18]. In the meta-analyses, a fixed-effect model was used when nonsignificant heterogeneity was observed (I2 < 50% and P > 0.1); otherwise, a random-effect model was selected to pool the effect size when significant between-study heterogeneity was found (I2 ≥ 50% or P ≤ 0.1). In the sensitivity analysis, the influence of an individual study on the pooled summary statistics was examined by omitting one study at a time and analyzing the pooled RR of the remaining studies. When possible, subgroup analyses were performed to assess the potential impacts of study design, location, positive control drugs and adjusting variables. Publication bias could be intuitively judged by funnel plot only when the number of included studies was over 10. Publication bias was quantitatively evaluated by Egger's linear regression test using Stata 12.0 software [19].
Results
Results of the literature search
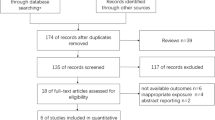
As with the initial step of the database search strategy, 237 relevant studies were identified and 52 duplicates were removed. After reviewing the titles and abstracts, ten full texts were included for detailed evaluation, of which five were excluded due to unavailable data (n = 3) and inappropriate exposure (n = 2). Eventually, five eligible studies [13, 14, 20,21,22] were included in our meta-analysis (Fig. 1), encompassing a case–control study [14], two prospective cohort studies [13, 20], and two retrospective cohort studies [21, 22]. Two studies were conducted in China [13, 20], one in the Republic of Korea [22], the United States of America [21], and the United Kingdom [14]. All studies were published from 2011 to 2019. In one study [21], T2DM patients in the control group received sulfonylurea derivatives, and in other four studies [13, 14, 20, 22], patients in the control group accepted non-metformin drugs. Basic information of the five included studies is listed in Table 1. Confounding variables of each study were adjusted for, and adjusted covariates are summarized in Table 2.
Methodological quality of included studies
The included studies were scored from 7 to 8 points (≥ 6 stars) using the NOS, which indicated that these studies were of high quality. The results of methodological quality assessment are shown in Table 3.
Overall meta-analysis
Based on the five studies [13, 14, 20,21,22], the meta-analysis using a random-effect model showed that metformin did not reduce EC risk in T2DM patients (HR 0.88, 95% CI 0.60–1.28, P > 0.05, Fig. 2), with moderate heterogeneity (P = 0.08 and I2 = 52%). The sensitivity analysis showed that the direction of the pooled effect of metformin on EC risk in T2DM patients was not influenced by any single study. The underlying heterogeneity was most likely attribute to the study by Becker [14]. After removing this study, the I2 statistic drastically dropped to 0% (P = 0.39). Therefore, a fixed-effect model was adopted for the meta-analysis based on the remaining four studies. Again, the results showed that metformin did not reduce EC risk in T2DM patients (HR 0.76, 95% CI 0.56–1.04, P > 0.05), as shown in Fig. 3.
Subgroup analyses
To explore the potential sources of between-study heterogeneity, subgroup analyses were performed regarding the study design, location, positive-control drugs and the adjustment for variables (Table 4). Subgroup analyses by location showed that metformin reduces EC risk in T2DM patients in Asian countries (HR 0.59; 95% CI 0.39–0.91; P = 0.02), without significant heterogeneity (P = 0.80 and I2 = 0%). In the subgroup analysis grouped by study design and positive-control drugs, no significant differences of reduced EC risks were found. After adjusting for smoking, BMI and serum HbA1c levels, a nonsignificant association was found between metformin use and EC risk in T2DM patients.
Publication bias
The Egger's test showed that there was no publication bias across studies (P = 0.232).
Discussion
Metformin as a first-line hypoglycemic drug for T2DM patients [23] can reduce blood glucose by inhibiting glycogenesis in hepatocytes and enhancing glucose uptake in peripheral tissues (such as muscles), with increased sensitivity of the liver to insulin [24, 25]. Since the study by Evans et al. [26] reports that metformin can significantly reduce the incidence rate of T2DM patients, more evidences support that metformin also serves as an agent for tumor prevention and therapy in T2DM patients. Preclinical and clinical studies even find it an effective anti-proliferation and pro-apoptotic drug [27,28,29].
The results of the present meta-analysis representing 528, 194 T2DM patients indicate that metformin does not reduce the risk of EC. Three [13, 14, 21] of the five studies have adjusted for smoking and BMI, and two [14, 21] for HbA1c, with no changes in the result of an overall meta-analysis.
The sensitivity analysis showed that the study by Becker et al. [15] had a considerable impact on the pooled effect. Though this study [15] has a weight far larger than other studies, the effect of metformin on EC risk in T2DM patients is not influenced. This may attribute to the type of a case–control study according to the study design, which can be a cause of heterogeneity. Besides, all but one study (Becker’s study [15]) has adopted the International Classification of Diseases (ICD) coding for cancer case ascertainment, suggesting that assessment methods can be another cause of heterogeneity.
Subgroup analyses showed that metformin significantly reduced EC risk in T2DM patients in Asian countries. Although the reasons behind this finding still remain uncertain, several possible explanations have been proposed according the following four dimensions. First, the demography of the type of EC, including squamous cell carcinoma and adenocarcinoma [1]. Esophageal squamous cell carcinoma (ESCC) is more prevalent in Asian populations, while esophageal adenocarcinoma (EAC) is the main type in western countries [1]. Along with an inhibitory effect of metformin on ESCC that has been found in both in vitro and in vivo experiments [30, 31], we speculate that the type-specific prevalence of EC among different races can be one of the reasons. Second, the particularly high-EC incidence in Asia [32]. The relatively low absolute number of EC incidents in Western countries may limit the accuracy of the risk assessment. Third, risk factor disparities among regions. Alcohol and smoking account for 90% of the cases in Western countries [33, 34]; while poor nutrition, low intakes of fruits and vegetables, hot beverages, salted and preserved food intakes are considered as high risk factors in Asia [35,36,37]. Three studies [13, 14, 21] have adjusted for smoking, but all included studies have not adjusted alcohol or dietary factors. Fourth, two major causes of EAC, obesity and gastroesophageal reflux disease, in Western countries [1]. Many obese people in Western countries are prone to have EC, who are the predominant population of T2DM patients and the main consumers of metformin. Therefore, EAC risk can be overestimated and thereby affect the accuracy of our results. Therefore, we speculate that different pathological types, impact of geographical prevalence, and risk factor disparities may be responsible for the efficacy difference of metformin in EC risk reduction among regions. To discuss whether the influence of metformin has been confounded with a good glycemic control, serum HbA1c levels in the Asian subgroup should have been adjusted for in their clinical trials (three included studies). However, none of the three studies we included in the Asian subgroup adjusted HbA1c level, this may interfere with the accuracy of the results. Therefore, more high-quality RCTs with adjustment for HbA1c levels are needed.
Evidences show that metformin exerts an anti-tumor effect with the involvement of multiple molecules and multiple metabolic pathways, increasing autophagy and apoptosis of tumor cells and inhibiting cell proliferation in tumor cells. The AMP-activated protein kinase (AMPK) signaling pathway is one of the classical mechanisms behind the anti-tumor effects of metformin, which is more potent in autophagy and apoptosis [38, 39]. In mammals, AMPK as a serine/threonine protein kinase composed of α catalytic subunits (α 1 and 2), β regulatory subunits (β 1 and 2) and γ regulatory subunits (γ 1, 2 and 3) mainly regulates cellular energy metabolism [40, 41]. Metformin is currently believed to activate the AMPK pathway by two means [42, 43]: one is to increase active nitrogen levels via blocking the transmission of mitochondrial respiratory chain complex I in cells, thus activating protein kinase C (PKC) and inducing AMPK phosphorylation; the other is to increase intracellular AMP levels, reduce ATP synthesis and directly activate the AMPK pathway. The key upstream activator, liver kinase B1 (LKB1), is considered to be a tumor suppressor, which is closely related to the activation of the AMPK pathway. When LKB1 mutates or loses its function, the anti-tumor activity of metformin also decreases, so it is considered to be an important regulator for tumor cell growth and metabolism [44].
To the best of our knowledge, this is the first meta-analysis to evaluate the effect of metformin on EC risk in T2DM patients. However, some limitations in our analysis must be clearly acknowledged. First, as the limited number of included studies and limitations in the basic methodology of observational studies, our findings should be regarded as hypothesis generation and exploratory. Second, statistical heterogeneity is high in our meta-analysis. Although sensitivity and subgroup analyses can explain some of the sources of heterogeneity, other underlying sources such as cumulative duration of metformin treatment and doses of metformin cannot be analyzed due to a lack of data. Third, there is a lack of uniformity in the variables adjusted in multivariate-adjusted risk estimates. None of the included studies have adjusted for alcohol and dietary factors, which are important risk factors for EC. Fourth, all included studies have failed to provide histopathological grading or staging systems of EC so that we are unable to further explore the effect of metformin among pathological types of EC. Finally, to better assess the efficacy of metformin, measurements of key biomarkers and patient compliance during the follow-up are of vital importance. However, as these parameters have not been provided, changes in antidiabetic drug regimens during the follow-up are unknown, which may affect the credibility of the results.
Conclusions
Overall, our systematic review and meta-analysis demonstrate that metformin does not reduce esophageal cancer risk in type 2 diabetes mellitus patients. However, a significant reduction in esophageal cancer risk in Asian populations remains to be clarified.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Smith U, Gale EM. Cancer and diabetes: are we ready for prime time? Diabetologia. 2010;53:1541–4.
Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and Cancer. Endocr Relat Cancer. 2009;16:1103–23.
Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871–7.
Huang W, Ren H, Ben Q, Cai Q, Zhu W, Li Z. (2012) Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control. 2012;23:263–72.
Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016. JAMA Oncol. 2018;4:1553–688.
Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018;143:409–19.
Shuai Y, Li C, Zhou X. The effect of metformin on gastric cancer in patients with type 2 diabetes: a systematic review and meta-analysis. Clin Transl Oncol. 2020. https://doi.org/10.1007/s12094-020-02304-y.
Nie Z, Zhu H, Gu M. Reduced colorectal cancer incidence in type 2 diabetic patients treated with metformin: a meta-analysis. Pharm Biol. 2016;54:2636–42.
Tseng CH. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer. 2014;50:2831–7.
Yeung SL, Schooling CM. Impact of glycemic traits, type 2 diabetes and metform in use on breast and prostate cancer risk: a Mendelian randomization study. BMJ Open Diabetes Res Care. 2019;7:e000872.
Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–8.
Tseng CH. Metformin and esophageal cancer risk in Taiwanese patients with type 2 diabetes mellitus. Oncotarget. 2016;8:18802–10.
Becker C, Meier CR, Jick SS, Bodmer M. Case–control analysis on metformin and cancer of the esophagus. Cancer Causes Control. 2013;24:1763–70.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in taiwanese: a representative population prospective cohort study of 800, 000 individuals. BMC Cancer. 2011;11:20–9.
Murff HJ, Roumie CL, Greevy RA, Hackstadt AJ, McGowan LED, Hung AM, et al. Metformin use and incidence cancer risk: evidence for a selective protective effect against liver cancer. Cancer Causes Control. 2018;29:823–32.
Oh TK, Song IA. Metformin use and the risk of cancer in patients with diabetes: a nationwide sample cohort study. Cancer Prev Res (Phila). 2020;13:195–202.
Witters LA. The blooming of the French lilac. J Clin Invest. 2001;108:1105–7.
Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–9.
Holland W, Morrison T, Chang Y, Wiernsperger N, Stith J. Metformin(Glucophage) inhibits tyrosine phosphatase activity to stimulate the insulin receptor tyrosine kinase. Biochem Pharmacol. 2004;67:2081–91.
Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5.
Wojciechowska J, Krajewski W, Bolanowski M, Kręcicki T, Zatoński T. Diabetes and cancer: a review of current knowledge. Exp Clin Endocrinol Diabetes. 2016;124:263–75.
Meyerhardt JA, Irwin ML, Jones LW, Zhang S, Campbell N, Brown JC, et al. Randomized phase II trial of exercise, metformin, or both on metabolic biomarkers in colorectal and breast cancer survivors. JNCI Cancer Spectr. 2019;4:pkz096.
Zhou HY, Yao XM, Chen XD, Tang JM, Qiao ZG, Wu XY. Mechanism of metformin enhancing the sensitivity of human pancreatic cancer cells to gemcitabine by regulating the PI3K/Akt/mTOR signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:10283–9.
Kobayashi M, Kato K, Iwama H, et al. Antitumor effect of metformin in esophageal cancer: in vitro study. Int J Oncol. 2013;42:517–24.
Feng Y, Ke C, Tang Q, et al. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis. 2014;5:e1088.
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. 2013;23:233–42.
Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235–56.
Matejcic M, Gunter MJ, Ferrari P. Alcohol metabolism and oesophageal cancer: a systematic review of the evidence. Carcinogenesis. 2017;38:859–72.
Trevellin E, Scarpa M, Carraro A, Lunardi F, Kotsafti A, Porzionato A, et al. Esophageal adenocarcinoma and obesity: peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget. 2015;6:11203–15.
Lin S, Wang X, Huang C, Liu X, Zhao J, Yu IT, et al. Consumption of salted meat and its interactions with alcohol drinking and tobacco smoking on esophageal squamous-cell carcinoma. Int J Cancer. 2015;137:582–9.
Liu X, Wang X, Lin S, Lao X, Zhao J, Song Q, et al. Dietary patterns and the risk of esophageal squamous cell carcinoma: a population-based case–control study in a rural population. Clin Nutr. 2017;36:260–6.
Green AS, Chapuis N, Maciel TT, Willems L, Lambert M, Arnoult C, et al. The LKBl/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNAtranslation. Blood. 2010;116:4262–73.
Grimaldi C, Chiarini F, Tabellini G, Ricci F, Tazzari PL, Battistelli M, et al. AMP-dependent kinase/mammalian target of rapamycin complex 1 signaling in T-cell acute lymphoblastic leukemia: therapeutic implications. Leukemia. 2011;26:91–100.
Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–85.
Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond). 2008;32:S55–S59.
El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–8.
Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33.
Lin SC. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27:299–313.
Acknowledgements
The authors are grateful to Yanhua Song for proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest to this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, HD., Zhang, JJ. & Zhou, BJ. The effect of metformin on esophageal cancer risk in patients with type 2 diabetes mellitus: a systematic review and meta‑analysis. Clin Transl Oncol 23, 275–282 (2021). https://doi.org/10.1007/s12094-020-02415-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02415-6