Abstract
Background
Metformin, a drug widely used in the treatment of diabetes, has proven preventive and survival benefits for various malignancies. However, the effect of metformin on gastric cancer risk and survival rate in T2DM patients remains controversial. Therefore, we conducted a systematic review and meta-analysis to evaluate the effect of metformin on gastric cancer in T2DM patients.
Methods
We searched PubMed, EMBASE, Medline and the Cochrane Library for related studies up to October 22, 2019. Pooled hazard ratios with 95% confidence intervals were calculated using random-effects model. Heterogeneity was assessed. All articles were evaluated by Newcastle–Ottawa Scale.
Results
A total of 11 cohort studies met eligibility criteria and were included in the meta-analysis. The use of metformin was related to a significant 21% reduction in GC incidence (HR 0.790; 95% CI 0.624–1.001). Subgroup analysis showed that the use of metformin significantly reduced the risk of gastric cancer in T2DM patients in Asian populations, but not in western populations. In a pooled analysis of 3 studies, metformin use was associated with increased overall survival rate (HR 0.817; 95% CI 0.600–1.113) and cancer-specific survival rate (HR 0.824; 95% CI 0.614–1.106) of T2DM patients.
Conclusions
Metformin could reduce the risk of gastric cancer in T2DM patients, particularly in Asian populations. However, it is debatable whether metformin use can improve the prognosis of gastric cancer in T2DM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fifth most common cancer in the world (World Health Organization 2012). This cancer has a poor prognosis with a 5-years survival rate of 25–30% [1], which is the third leading cause of cancer-related deaths worldwide (World Health Organization 2012), with 723,000 deaths (8.8% of all cancer deaths) in a year. The current treatment is mainly surgery combined with traditional chemotherapy [2].
The prevalence of type 2 diabetes (T2DM) is predicted to increase from 2.8 in 2000 to 4.4% in 2030 [3]. From previous epidemiological studies, we have found that T2DM patients have a significant increase in cancer risk and mortality compared to non-diabetic patients [4,5,6,7,8,9].
Metformin, one of the biguanide classes, is a widely used drug for the treatment of diabetes. In previous meta-analyses, the survival benefits of metformin have been demonstrated in a variety of malignancies, including breast, prostate, pancreatic, colorectal, and lung cancer [10,11,12,13,14]. For GC, metformin can inhibit the proliferation of tumor cells in vivo and in vitro [15, 16].
Currently, the effect of metformin on GC risk and survival rate in T2DM patients remains controversial. Previous meta-analyses have suggested that metformin appears to play a protective role in the development of GC in T2DM patients, but the results are limited [17, 18], and new studies published recently did not support that conclusion [19,20,21].
Considering this controversial issue, and the prognostic significance and survival outcomes of metformin in T2DM patients with GC have not been systematically evaluated, we conducted a systematic review and meta-analysis including recent cohort studies to evaluate whether metformin can prevent the development of GC in T2DM patients and whether metformin can improve overall survival (OS) and cancer-specific survival (CSS) of T2DM patients with GC.
Methods
Search strategy
We performed a search of PubMed, EMBASE, Medline and the Cochrane Library for studies published up to October 22, 2019. The databases were searched based on a combination of the following words: (“Stomach Neoplasms” OR “Neoplasm, Stomach” OR “Stomach Neoplasm” OR “Neoplasms, Stomach” OR “Gastric Neoplasms” OR “Gastric Neoplasm” OR “Neoplasm, Gastric” OR “Neoplasms, Gastric” OR “Cancer of Stomach” OR “Stomach Cancers” OR “Gastric Cancer” OR “Cancer, Gastric” OR “Cancers, Gastric” OR “Gastric Cancers” OR “Stomach Cancer” OR “Cancer, Stomach” OR “Cancers, Stomach” OR “Cancer of the Stomach” OR “Gastric Cancer, Familial Diffuse”) and (“Metformin” OR “Dimethylbiguanidine” OR “Dimethylguanylguanidine” OR “Glucophage” OR “Metformin Hydrochloride” OR “Hydrochloride, Metformin” OR “Metformin HCl” OR “HCl, Metformin”). No language restrictions were applied. We also performed a manual search of the references from selected articles and reviews which related to our research to identify additional relevant studies. The investigation was conducted independently by two investigators and differences were resolved through discussion.
Study selection
Studies were selected if they met the following criteria: [1] original full-text studies that were designed to evaluate the effect of metformin on GC in T2DM patients; [2] studies which comprised an observation group that received metformin therapy and a control group that received other antidiabetic drugs; [3] the association between metformin and GC was assessed using hazard ratio (HR) or adjusted HR and 95% confidence intervals (CIs); [4] retrospective or prospective cohort studies, randomized clinical trials, and case–control studies. When multiple publications came from the same population, the most recent or comprehensive one was given precedence.
The following studies were excluded from the meta-analysis: [1] duplicate studies; [2] studies based on cell or animal models; [3] letters, reviews, comments, and conference abstract; [4] studies lacking relevant outcomes.
Quality assessment
The quality of the included studies was assessed using the Newcastle–Ottawa scale (NOS), which judges the selection of the study groups according to three domains: selection, comparability and outcome [22]. The full score was 9 stars, and studies with a cumulative score ≥ 7 (NOS scores = 7, 8, 9) were defined as high-quality studies. Studies scoring 4–6 were defined as moderate-quality studies, and studies scoring 3 or below were defined as low-quality studies.
Data extraction
The following data were extracted from eligible articles by two researchers into a standard spreadsheet: (a) author names; (b) country of origin; (c) year of publication; (d) study design; (e) number of patients with or without metformin use; (f) patient age; g) follow-up time; h) treatment in the control group; (i) adjusted hazard ratio (AHR) and 95% CI; (j) adjustment variables. Any disagreements over the retrieved information were resolved by consensus, referring back to the original articles.
Statistical analysis
Pooled HRs with 95% CI were analyzed using a random-effects model as substantial interstudy heterogeneity existed for most outcomes [23]. We assessed the statistical heterogeneity among the summary data by the I2 statistic and the Cochran’s Q statistic. For the Q statistic, P < 0.05 was considered statistically significant for heterogeneity. For the I2 statistic, 25–50% is regarded as low heterogeneity; a value of > 50% as the standard of significant heterogeneity [24]. We conducted sensitivity analyses to assess the robustness of results. Subsequently, we conducted subgroup analysis for the risk of GC and metformin use in T2DM patients to assess the sources of heterogeneity more accurately: [1] study location; [2] control drugs; [3] adjustment variables. We did not generate funnel plots because there were fewer than 10 studies in each group [25,26,27]. Due to the lack of articles about the impact of metformin on survival outcomes in T2DM patients with GC (n = 3), we did not perform relevant subgroup analysis. Two investigators analyzed the data independently. All statistical analyses were conducted using Stata software (version 13.0; Stata Corporation, College Station, Tex).
Results
Search results
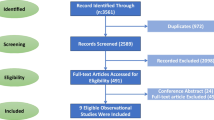
A total of 327 citations were identified using PubMed, EMBASE, Medline and the Cochrane Library. Of these, 99 duplicate studies were excluded. After screening title and abstract, 147 studies were excluded, and the remaining 81 articles were retrieved for full-text review. Then, we excluded 70 articles which did not meet the inclusion criteria. Of these articles, 36 were excluded because of their publication type, 27 because they were animal or laboratory studies, and 4 because they did not have relevant outcomes. In addition, Chen et al. [28], Lee et al. [29] and Tseng et al. [30] assessed the same population from the Taiwan National Health Insurance Research Database (NHIRD), and the populations in Ruiter et al.’s study [31] and de Jong et al.’s study [32] were both from the Netherlands Cancer Registry (NCR)-PHARMO database. To avoid the overlap of the patient population, three overlapping studies were excluded [28, 29, 31]. Finally, 11 cohort studies [15, 19, 20, 30, 32,33,34,35,36,37,38], were included in our overall analysis of the effect of metformin on GC in T2DM patients. No additional articles from the references were added to this review. A flowchart of the selection process for this study is presented in Fig. 1.
Study characteristics
The characteristics of the included studies and patients are summarized in Table 1. The selected studies were all cohort studies published in the past 5 years (2014–2019). Of the included studies, five were conducted in Asia (China, Republic of Korea), and five in Europe (Italy, Netherlands, Sweden, UK, Lithuania). The remaining were from the United States. The case sources of the included studies were all population based. Among the included articles, eight studied the effect of metformin on the risk of GC in T2DM patients, and the remaining three studied the effect of metformin on survival of T2DM patients with GC. In seven studies, the treatment of the control group was T2DM patients taking non-metformin drugs; in two studies, the control group was T2DM patients taking non-insulin antidiabetic drugs; and in the remaining two, the control group was treated with Sulfonylurea derivatives. Different confounding variables were adjusted in each study (Table 2), such as age, sex, race, date of cohort entry, body mass index (BMI), blood pressure, glomerular filtration rate, hemoglobin A1c, low-density lipoprotein levels, smoking status, select medications, number of medications, number of outpatient visits, etc.
The quality assessment with reference to the Newcastle–Ottawa statement is shown in Table 3. On the whole, the studies achieved relatively high scores ranging from 7 to 9, which indicated that these studies were of high quality.
Overall analysis
On meta-analysis of 8 included studies (1,238,382 patients) assessing the relationship between the risk of GC and metformin use in T2DM patients, pooled HRs and corresponding 95% CIs are shown in Fig. 2. The use of metformin has been shown to be related to a significant 21% reduction in GC incidence. (HR 0.790; 95% CI 0.624–1.001; P = 0.051). Heterogeneity was significant between the studies (I2 = 88.3% and P < 0.001). We excluded one study at a time and recalculated the combined HR of the rest of the study to perform sensitivity analyses. Sensitivity analyses demonstrated that the removal of any individual article had no substantial effect on the overall results, but when we excluded Cheung et al., the Heterogeneity reduced (I2 = 40.6% and P = 0.12).
And the estimated HRs for association between GC survival rate and exposure to metformin for each study are shown in Figs. 3 and 4. In a pooled analysis of 3 studies (707 patients), the use of metformin is associated with increased overall OS rate (HR 0.817; 95% CI 0.600–1.113; P = 0.201) and cancer-specific CSS rate (HR 0.824; 95% CI 0.614–1.106; P = 0.197) of T2DM patients. Low heterogeneity was observed between the studies. (I2 = 38.2%, P = 0.198; I2 = 23.3%, P = 0.271, respectively). As there was only one study regarding the effect of metformin on the recurrence-free survival (RFS) of GC, a meta-analysis on this was not conducted.
Subgroup analysis
We performed subgroup analysis to explore the potential heterogeneity among the articles and the effect of these characteristics on the summary results (Table 4). Stratified analyses by location found that Asian showed a significant association between the use of metformin and the risk of GC in people with T2DM (HR 0.54; 95% CI 0.38–0.78; P = 0.001), with high heterogeneity (I2 = 67.1%). Although there were significant differences among Western, metformin use could hardly reduce the incidence of gastric cancer in T2DM patients (HR 0.99; 95% CI 0.99–0.99; P < 0.001). In addition, the Western group had no heterogeneity (I2 = 0). In the subgroup analysis grouped by control drugs, no significant differences were found. After adjusting for BMI, age and H. pylori eradication therapy, no vital association was found between metformin use and the risk of GC in T2DM patients. In terms of Hemoglobin A1c, the use of metformin was related to a statistically significant 37% reduction in GC incidence (HR 0.63; 95% CI 0.61–0.97; P = 0.034), with low heterogeneity (I2 = 2.1%).
Discussion
Metformin is the most common first-line treatment for T2DM. Previous epidemiological studies have shown that diabetics treated with metformin have a significantly lower risk of developing cancer than those who are not [21, 39]. The anti-cancer activity by metformin is proposed to be mediated by two pathways. First, metformin is an insulin sensitizer, which reduces the production of insulin and insulin growth factors (IGFs). The IGFs signaling pathway can stimulate the proliferation of cancer cells expressing IGF receptors [40]. Second, metformin has also direct antiproliferative effects, through the activation of 5′-adenosine monophosphate activated protein kinase (AMPK), metformin inhibits the expression of mammalian target of rapamycin (mTOR), which in turn prevents cell aging and cancer development [41]. The upstream regulator of AMPK is the protein kinase LKB1, which is a well-recognized tumor suppressor.
Observational studies have suggested a possible relationship between treatment with metformin and decreased incidence of cancer in participants with T2DM. Currently, some experiments have shown that metformin can inhibit the proliferation of cancer cells in cell culture [16, 42,43,44]. However, a randomized controlled trial did not support the previous results. Home PD et al. extracted data for malignancies from the ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) randomized controlled clinical trials, in which the efficacy and/or safety of metformin was assessed in comparison with sulfonylureas and rosiglitazone. The results suggested that metformin did not offer any particular protection against malignancy compared with rosiglitazone [45].
Our meta-analysis of 8 observational cohort studies showed that metformin could reduce the risk of GC in T2DM patients and the results were of borderline statistical significance (P = 0.051). Three more trials studying the relationship between metformin use and GC survival outcomes shown different results. Lee et al. [15] shown that T2DM patients with GC who received metformin after gastrectomy had a better prognosis than those who did not (OS: HR 0.584 [95% CI 0.369–0.926], CSS: HR 0.57 [95% CI 0.334–0.975], RFS: HR 0.633 [95% CI 0.410–0.977]), and the efficacy of metformin was proportional to the cumulative duration of use. The results of Baglia et al. [33] and Dulskas et al. [19] showed that the use of metformin had no positive effect on the survival rate of T2DM patients with GC. In addition, the study of Lacroix et al. [46] did not adjust the type of diabetes suggesting that metformin use might improve overall mortality. However, no such association was found for cancer-specific survival. In view of this problem, on the one hand, the studies were based on different populations, and the effect of population differences on the efficacy of metformin is still unclear. On the other hand, we hope that in the future, more relevant studies will be conducted to explore the effect of metformin use on the prognosis of T2DM patients with GC, so as to help clinicians provide more targeted treatment to patients and improve their quality of life.
In our opinion, previous meta-analyses had some limitations. In the article of Zhou et al. [17], the four articles from Taiwan used the same database, which might result in patient duplication. Li et al. [18] only reported a systematic review about metformin use and its effect on GC in T2DM patients.
From the data, it seems that different study populations have significant effects on metformin efficacy. Zheng et al. [38] indicated that metformin use did not decrease the risk of gastric cancer in a Western population during the follow-up of a median of 6 years. Our subgroup analysis showed that metformin use significantly reduced the risk of gastric cancer in T2DM patients in the Asian population (HR 0.54; 95% CI 0.38–0.78; P = 0.048); however, there is no evidence of protective effect of metformin in the western population (HR 0.99; 95% CI 0.99–0.99; P < 0.001), which is consistent with previous reports. As for hemoglobin A1c, a population-based cohort study shown that GC risk was higher among individuals with higher hemoglobin A1c levels [47]. However, Cheung et al. [36] categorized the time-weighted average HbA1c into a binary variable by a cut-off value of 7% and found a higher time-weighted average HbA1c level of at least 7% was not an independent risk factor for GC after H. pylori eradication for T2DM patients. Due to the lack of relevant trials, more relevant trials could help to better understand the effect of hemoglobin A1c levels on GC risk in the future.
Meanwhile, there are several limitations in this meta-analysis that should be acknowledged. First, due to the limited number of included articles, we did not use funnel plots to evaluate publication bias, which may also be a part of the source of high heterogeneity. Second, there are some methodological shortcomings in observational articles, which are prone to time-related biases, such as immortal time bias and time-lagging issues [48]. Third, adjustment variables of included studies were inconsistent and incomplete. Helicobacter pylori is known to be an important cause of GC, but only two studies have adjusted for this confounding factor. In our study, for example, information on BMI, follow-up time, use time of metformin, regular exercise, dietary habits, other treatments (chemotherapy, radiotherapy, surgery) would have been important to adjust for residual confounding, and insufficient adjustment for important confounding factors may lead to decreased accuracy of results. Finally, 4 articles were rejected without relevant results; a selection bias might occur.
In conclusion, our study suggested that metformin might reduce the risk of gastric cancer in T2DM patients, particularly in Asian populations. However, it is debatable whether metformin use can improve the prognosis of gastric cancer in T2DM patients. Considering our limitations and the heterogeneity among the studies, relevant randomized controlled trials and more well-designed prospective cohort studies are expected.
References
Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40(3):250–60.
Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(10):1286–312.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53.
Adami HO, McLaughlin J, Ekbom A, Berne C, Silverman D, Hacker D, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control. 1991;2(5):307–14.
La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994;70(5):950–3.
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–85.
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159(12):1160–7.
Rousseau MC, Parent ME, Pollak MN, Siemiatycki J. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal Canada. Int J Cancer. 2006;118(8):2105–9.
Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S, et al. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166(17):1871–7.
Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist. 2013;18(12):1248–55.
He XK, Su TT, Si JM, Sun LM. Metformin is associated with slightly reduced risk of colorectal cancer and moderate survival benefits in diabetes mellitus: a meta-analysis. Med (Baltimore). 2016;95(7):e2749.
Stopsack KH, Ziehr DR, Rider JR, Giovannucci EL. Metformin and prostate cancer mortality: a meta-analysis. Cancer Causes Control. 2016;27(1):105–13.
Xu H, Chen K, Jia X, Tian Y, Dai Y, Li D, et al. Metformin use is associated with better survival of breast cancer patients with diabetes: a meta-analysis. Oncologist. 2015;20(11):1236–44.
Wan G, Yu X, Chen P, Wang X, Pan D, Wang X, et al. Metformin therapy associated with survival benefit in lung cancer patients with diabetes. Oncotarget. 2016;7(23):35437–45.
Lee CK, Jung M, Jung I, Heo SJ, Jeong YH, An JY, et al. Cumulative metformin use and its impact on survival in gastric cancer patients after gastrectomy. Ann Surg. 2016;263(1):96–102.
Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, et al. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012;11(3):549–60.
Zhou XL, Xue WH, Ding XF, Li LF, Dou MM, Zhang WJ, et al. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: a meta-analysis of cohort studies. Oncotarget. 2017;8(33):55622–31.
Li P, Zhang C, Gao P, Chen X, Ma B, Yu D, et al. Metformin use and its effect on gastric cancer in patients with type 2 diabetes: a systematic review of observational studies. Oncol Lett. 2018;15(1):1191–9.
Dulskas A, Patasius A, Linkeviciute-Ulinskiene D, Zabuliene L, Smailyte G. A cohort study of antihyperglycemic medication exposure and survival in patients with gastric cancer. Aging (Albany NY). 2019;11(17):7197–205.
Valent F. Diabetes mellitus and cancer of the digestive organs: an Italian population-based cohort study. J Diabetes Complicat. 2015;29(8):1056–61.
Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila). 2010;3(11):1451–61.
Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Higgins J. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011; www.cochrane-handbook.org.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis: prevention, assessment and adjustments. Psychometrika. 2007;72(2):269.
Chen YL, Cheng KC, Lai SW, Tsai IJ, Lin CC, Sung FC, et al. Diabetes and risk of subsequent gastric cancer: a population-based cohort study in Taiwan. Gastric Cancer. 2013;16(3):389–96.
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC, et al. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20.
Tseng CH. Metformin reduces gastric cancer risk in patients with type 2 diabetes mellitus. Aging (Albany NY). 2016;8(8):1636–49.
Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, Geelhoed-Duijvestijn PH, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care. 2012;35(1):119–24.
De Jong RG, Burden AM, de Kort S, van Herk-Sukel MP, Vissers PA, Janssen PK, et al. No decreased risk of gastrointestinal cancers in users of metformin in The Netherlands; a time-varying analysis of metformin exposure. Cancer Prev Res (Phila). 2017;10(5):290–7.
Baglia ML, Cui Y, Zheng T, Yang G, Li H, You M, et al. Diabetes medication use in association with survival among patients of breast, colorectal, lung, and gastric cancer. Cancer Res Treat. 2019;51(2):538–46.
Kim YI, Kim SY, Cho SJ, Park JH, Choi IJ, Lee YJ, et al. Long-term metformin use reduces gastric cancer risk in type 2 diabetics without insulin treatment: a nationwide cohort study. Aliment Pharmacol Ther. 2014;39(8):854–63.
Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, et al. Metformin does not affect cancer risk: a cohort study in the U.K. clinical practice research datalink analyzed like an intention-to-treat trial. Diabetes Care. 2014;37(9):2522–32.
Cheung KS, Chan EW, Wong AYS, Chen L, Seto WK, Wong ICK, et al. Metformin use and gastric cancer risk in diabetic patients after helicobacter pylori eradication. J Natl Cancer Inst. 2019;111(5):484–9.
Murff HJ, Roumie CL, Greevy RA, Hackstadt AJ, McGowan LED, Hung AM, et al. Metformin use and incidence cancer risk: evidence for a selective protective effect against liver cancer. Cancer Causes Control. 2018;29(9):823–32.
Zheng J, Xie SH, Santoni G, Lagergren J. Metformin use and risk of gastric adenocarcinoma in a Swedish population-based cohort study. Br J Cancer. 2019;121(10):877–82.
Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–5.
Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–28.
Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK. Metformin: Taking away the candy for cancer? Eur J Cancer. 2010;46(13):2369–80.
Yu G, Fang W, Xia T, Chen Y, Gao Y, et al. Metformin potentiates rapamycin and cisplatin in gastric cancer in mice. Oncotarget. 2015;6(14):12748–62.
Han G, Gong H, Wang Y, Guo S, Liu K. AMPK/mTOR-mediated inhibition of survivin partly contributes to metformin-induced apoptosis in human gastric cancer cell. Cancer Biol Ther. 2015;16(1):77–87.
Choi SI, Yoon C, Park MR, Lee D, Kook MC, Lin JX, et al. CDX1 expression induced by CagA-expressing helicobacter pylori promotes gastric tumorigenesis. Mol Cancer Res. 2019;17(11):2169–83.
Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti G, et al. Experience of malignancies with oral glucose-lowering drugs in the randomized controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia. 2010;53(9):1838–45.
Lacroix O, Couttenier A, Vaes E, Cardwell CR, De Schutter H, Robert A, et al. Impact of metformin on gastric adenocarcinoma survival: a Belgian population-based study. Cancer Epidemiol. 2018;53:149–55.
Ikeda F, Doi Y, Yonemoto K, Ninomiya T, Kubo M, Shikata K, et al. Hyperglycemia increases risk of gastric cancer posed by Helicobacter pylori infection: a population-based cohort study. Gastroenterology. 2009;136(4):1234–41.
Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35(12):2665–73.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shuai, Y., Li, C. & Zhou, X. The effect of metformin on gastric cancer in patients with type 2 diabetes: a systematic review and meta-analysis. Clin Transl Oncol 22, 1580–1590 (2020). https://doi.org/10.1007/s12094-020-02304-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02304-y