Abstract
Purpose
Immunotherapy is a new standard first-line treatment for non-small cell lung cancers (NSCLC) with high programmed cell death-ligand 1 (PD-L1) expression (≥ 50%) and second-line treatment regardless of PD-L1 status, though not all patients benefit from this approach. Much effort is ongoing to identify robust prognostic and predictive biomarkers of response to immune checkpoint inhibitors, overcoming PD-L1 that appears limited in its ability to discriminate patient candidates to this new class of anticancer agents. The purpose of this research study is to identify potential new biomarkers for immunotherapy in lung cancer.
Methods
Fifty-three consecutive patients with advanced NSCLC treated with nivolumab were enrolled in the study. All the patients received a blood analysis looking for the relationship between different populations of baseline white blood cells and granulocytic myeloid-derived suppressor cells (Gr-MDSC) detected by flow cytometry, to identify and characterize patients with poor likelihood of benefit from nivolumab in NSCLC second-line setting, regardless of clinical feature and PDL1 expression.
Results
Univariate analysis showed that high baseline levels of Gr-MDSC and low baseline CD8/Gr-MDSC ratio are associated with significantly better (P = 0.02) response to immunotherapy treatment. Log-rank tests suggested a significant improvement in OS and PFS with high baseline levels of Gr-MDSC levels (≥ 6 cell/μl), low absolute neutrophil count (< 5840/μl), high eosinophil count (> 90 /μl), and NLR < 3. The multivariate analysis showed a statistically significant improvement for PFS (P = 0.003) and OS (P = 0.05) in favour of the identified good prognostic Gr-MDSC-linked asset group, compared with the poor prognosis group.
Conclusion
The role of Gr-MDSC appears interesting as a potential biomarker in NSCLC patients receiving immune-checkpoint inhibitors. Further analyses are needed to confirmed and study in deep the role of these particular cells and their role in cancer response and progression during ICI therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is still the leading cause of cancer-related death in [1]. Despite progress in diagnostics, most new cases are in the advanced stages of the disease (57%) and have a poor 5-year overall survival (OS) rate (< 5%) [2]. In recent years, novel non-small cell lung cancer (NSCLC) treatments have been introduced that have revolutionized the therapeutic landscape for advanced stages showing predictive biomarker of response [2]. Programmed cell death (PD)-1 is an immune inhibitory receptor in the cluster of differentiation (CD)-28 protein family that is expressed on T cells, B cells, monocytes, natural killer (NK) cells, and many tumour-infiltrating lymphocytes [3]. Because various cancers, including lung cancer, express PD-ligand 1 (PD-L1), development of anti-PD-1 and anti-PD-L1 agents has been a major focus in the field of cancer immunotherapy [4]. Use of immune checkpoint inhibitors or immunotherapies has produced statistically and clinically significant improvements in OS versus second-line chemotherapy in most cases [5]. Whereas the efficacy of nivolumab and atezolizumab is independent of PD-L1, pembrolizumab requires at least 1% or more PD-L1 expression. On the other hand, pembrolizumab can produce an OS gain (HR = 0.6) over first-line platinum-based chemotherapy in NSCLC patients with strong PD-L1 expression (≥ 50%; about 25% of all NSCLC cases), achieving an unprecedented median survival of 30 months [5]. Thus, as a direct or indirect target of immune checkpoint inhibitors, PD-L1 may represent an excellent predictive factor for selecting patients who will benefit the most from immunotherapy, despite presents different limits.
However, even though immunotherapy can produce great and long-lasting results in both squamous and non-squamous histological types, not all NSCLC patients seem to benefit from this approach. Clinical trials using immune checkpoint inhibitors in NSCLC have shown that about 10% of patients with negative PD-L1 expression show a response to treatment, while the not well identifies a group of patients with moderate to strong PD-L1 expression (≥ 50%) do not, sometimes developing a rapid progression [5]. Dynamic expression of PD-L1, PD-L1 heterogeneity, different anti-PD-L1 antibodies, and expression of other checkpoint molecules that could cause resistance to given immunotherapy could partly explain the pitfalls and inaccuracies associated with using PD-L1 as a predictor of clinical benefit to immune checkpoint inhibitors [6].
Another critical predictive factor for response to immunotherapy is represented by tumour mutational burden (TMB), evaluated on tissue or plasma. Nowadays, data about TMB are intrigued but not yet mature and robust for an accurate and safe clinical practice [7]. Many trials are evaluating the predictive role of TMB but the available results need further confirmation in prospective randomized trials after the resolution of several issues [7,8,9,10,11], such as the definition of the threshold of mutations and understanding what type of mutations to count [12] Furthermore, novel biomarkers are needed that are able to better define patient populations that will respond to and benefit from immunotherapy.
Multiple investigations are now ongoing to identify novel predictive and prognostic circulating biomarkers to immunotherapy. Previous studies have shown that the baseline neutrophil-to-lymphocyte ratio (NLR) is associated with clinical outcome in several cancers, including NSCLC [13]. Several retrospective analyses demonstrated that immunotherapy (ipilimumab and/or nivolumab) can affect absolute blood cell counts and specific blood cell subpopulations in patients with melanoma and NSCLC, and some studies have shown that these changes may be associated with clinical outcome [14]. Recently, an eight-gene effector T cell (Teff) gene expression (or Teff/interferon γ) signature was investigated in the POPLAR trial (randomized phase II trial of atezolizumab versus docetaxel for patients with previously treated NSCLC). Even though this signature failed to predict PFS or best ORR, a statistical trend of longer OS in patients treated with atezolizumab was found [15]. In the present study, the role of baseline peripheral blood cell counts in NSCLC patients was investigated in relation to clinical outcomes following second-line nivolumab treatment.
Materials and methods
The current prospective study included 53 consecutive patients with advanced NSCLC. Patients started nivolumab (3 mg/kg intravenously infused over 1 h every 2 weeks) at the European Institute of Oncology (Milan, Italy) from October 2014 to March 2017. Inclusion criteria were histologically or cytologically confirmed stage IIIB (locally advanced or recurrent and not a candidate for definitive multimodal therapy) or stage IV NSCLC; aged 18 years or older; refractory to prior first-line platinum-based chemotherapy; negative for epidermal growth factor receptor, anaplastic lymphoma kinase, or c-ros oncogene 1; measurable disease by Response Evaluation Criteria In Solid Tumors version 1.1 prior to administration of nivolumab [16]; adequate organ function (cardiac, hematological, liver, and renal); able and willing to comply with the study protocol at all times. Patients were excluded from our analysis if they received nivolumab as part of a previous clinical trial. For patients who had the tumour tissue tested for PD-L1 expression as part of routine clinical care, we used the commercially available Dako 28-8 immunohistochemical assay (Dako, Carpinteria, CA, USA). This research was approved by the Institutional Ethics Committee and was carried out according to the Ethical Principles of the Declaration of Helsinki (3388B), and all patients provided written informed consent prior to study inclusion.
Immunophenotypic analysis of circulating cells
Blood samples (6 ml) were collected at baseline (pretreatment), 3 months after treatment onset, and the time at which disease progression was detected (if applicable) or after the 30th cycle of nivolumab (for patients still receiving treatment). Multicolour flow cytometry was used to evaluate different circulating leucocyte subpopulations that were identified after selection of CD45+ (pan leucocyte marker) cells and focusing on T cells, regulatory T cells (Treg), and monocytic (Mo)- and Gr-MDSCs. Following a 20-min incubation at 4 °C with monoclonal antibodies and lysis of red blood cells with an ammonium chloride solution, cells were resuspended in 500 µl of buffer for immunophenotypic analysis. After the acquisition of a minimum of 5 × 105 cells by flow cytometer (Navios Flow Cytometer, Beckman Coulter), data were analyzed with Kaluza Analysis Software (Beckman Coulter). The following lymphocytic subpopulations were detected: Treg (CD3+/CD4+/CD25hi+/CD127LOW/NEG), T lymphocytes (CD3+/CD8+, CD3+/CD4+, CD3+/CD8+/PD-1+, and CD3+/CD4+/PD-1+), B lymphocytes (CD22+/CD3−), NK dim cells (CD16+/CD56+), NK bright cells (CD56++), and NK-like T cells (CD3+/CD56+). MDSC subpopulations were identified as immature MDSC (SSClow/Lin−/HLA-DR−/LOWCD33+/CD13+/CD11b+/CD15−/CD14−), Gr-MDSC (SSClow Lin−/HLA-DR−/LOW CD33+/CD13+/CD11b+/CD15 + /CD14−), and Mo-MDSC (SSClow Lin−/HLA-DR−/LOW CD33+/CD13+/CD11b+/CD15−/CD14+). The gating strategy used for MDSC analysis is shown in Fig. 1.
PD-L1 detection in tissue and plasma
Plasma samples were collected and centrifuged for 10 at 1640 g and then split into 1-ml tubes and stored at − 20 °C. PD-L1 was detected using a Quantikine enzyme-linked immunosorbent assay human/cynomolgus monkey B7-H1/PDL1 kit according to the manufacturer’s instructions (R&D Systems). Tumour PD-L1 protein expression was assessed in pretreatment tumour biopsy specimens with a PD-L1 28-8 pharmDx immunohistochemical assay (Dako, Agilent, Santa Clara, CA, USA) according to the manufacturer’s instructions. Tumour PD-L1 expression was confirmed when staining of the tumour cell membrane (at any intensity) was observed at prespecified expression levels of 1% or higher in a section that included at least 100 tumour cells that could be evaluated. PD-L1 expression was evaluated in both tumour and inflammatory cells.
Statistical analysis
Medians and IQR (interquartile range) of circulating cell subpopulations (biomarkers) analyzed before and during treatment included Treg; CD3+CD4+, CD3+CD4+PD1+, CD3+CD8+, and CD3+CD8+PD1+ T lymphocytes; NK bright and dim cells; NK-like T cells; Gr-, Mo-, and i-MDSCs; absolute neutrophil and eosinophil counts; NLR; and circulating PD-L1 expression. OS was calculated from the date of the first nivolumab treatment to death (event) or last follow-up (censored). PFS was calculated from the date of first nivolumab treatment to the appearance of first recurrence or death (event), or last follow-up (censored). OS and PFS curves were estimated with the Kaplan–Meier method. Response evaluation was assessed according to Response Evaluation Criteria in Solid Tumors (version 1.1) as complete response, partial response, stable disease, and progressive disease. ORR was defined as the sum of complete and partial responses, and disease control rate was the sum of complete response, partial response, and stable disease. The associations of biomarkers at baseline with patient and disease characteristics and of changes in time with clinical ORR were analyzed using Wilcoxon signed rank test. Associations of biomarkers with OS and PFS were evaluated by log-rank test; patients were categorized considering marker median values. Biomarkers found to be associated with disease progression or survival in univariate analyses were used to identify features of patients with better prognosis. Multivariate Cox proportional hazards regression models were used to identifying the circulating cell subpopulations significantly associated with PFS and/or OS independent of other prognostic factors and confounders. Forward and backward selections were used to choose biomarkers to be included in multivariable models. Fully adjusted HRs with 95% CIs were reported. All analyses were carried out with R 3.4 and SAS software version 9.3 (SAS Institute, Cary, NC, USA). All reported tests were two sided, and a P < 0.05 was considered significant.
Results
From October 2014 to March 2017, a homogenous group of 53 consecutive patients with advanced or metastatic NSCLC were enrolled and treated with 3 mg/kg nivolumab every 15 days. Main characteristics of enrolled patients are summarized in Table 1. The median age was 64 years old (range 56–70 years), and there were 33 (62%) males. Forty (75%) patients had lung adenocarcinoma, and 13 (25%) had squamous cell carcinoma. Fourteen (26%) of the 53 patients had controlled brain metastasis without the need of concomitant steroid therapy; overall, the survival of these 14 patients was similar to that of the overall study population. Eighteen (34%) of the 53 patients had archival tumour tissue and were analyzed for PD-L1 expression. PD-L1 expression on inflammatory and tumor cells was categorized as follows: PD-L1 expression < 1% (n = 11, 21%); PD-L1 expression ≥ 1% (n = 7, 13%); no PD-L1 expression (n = 35, 66%). PD-L1 expression on tumor cells was distributed as follows: PD-L1 < 5% (n = 15, 28%) patients; PD-L1 ≥ 5% (n = 3, 6%); missing (n = 35, 66%), respectively. The median follow-up was 19 months (interquartile range 14–21 months). The ORR was 25% (13 patients); 1 (2%) achieved complete response and 10 (19%) showed a partial response. The disease control rate was 38% (20 patients), 7 of whom (13%) acquired stable disease as the best response to nivolumab. The median PFS and OS in all 53 patients were 2.49 months (95% CI 1.83–6.13) and 6.59 months (95% CI 4.197–10.59), respectively.
Correlation between cellular immunophenotype and clinical outcome
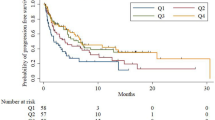
Univariate analyses of different immune markers showed a significant correlation with clinical response. In particular, higher baseline levels of granulocytic myeloid-derived suppressor cells (Gr-MDSC) were associated with a better ORR, as 9 patients with higher ORRs had significantly more (P = 0.02) Gr-MDSC [median = 10, interquartile range (IQR): 9–30] compared to the 32 stable and progressive disease patients (median = 4.5, IQR: 2.5–9.5, P = 0.019; Figs. 2, 3). Moreover, a low baseline CD8/Gr-MDSC ratio was associated with significantly better (P = 0.02) response to treatment; the median ratio in the 9 patients with higher ORRs was 16.8 (IQR: 16.0–45.6) compared to 68.55 in the 32 stable and progressive disease patients (IQR: 36.9–131.8, P = 0.017; Table 2. We also found significantly higher (P = 0.05) baseline circulating PD-L1 levels in patients with ORR and significantly lower (P = 0.02) of CD22+CD3− B lymphocytes (Table 2). Log-rank tests suggested significant improvement in OS and PFS with baseline levels of high Gr-MDSC levels, low absolute neutrophil count, high eosinophil count, NLR < 3, Eastern Cooperative Group Performance Status (ECOG PS) 0–1, and positive tissue PD-L1 expression. Patients with an ECOG PS of 2 or higher had poorer survival compared to patients with an ECOG PS of 0–1 (PFS HR = 0.21, P = 0.002; OS HR = 0.28, P = 0.002).
The results of multivariate Cox regression models adjusted for age, sex, and ECOG PS used to evaluate and compare the two different groups according to prognosis (good versus poor) showed a significant improvement in PFS (P = 0.003) and OS (P = 0.05) in favour of good versus poor prognosis group. These data were then stratified by patient population, baseline levels of Gr-MDSC levels, eosinophil count, neutrophils count, and NLR < 3 and showed a statistically significant difference between the good and poor prognosis immunological asset. For the immunological asset described in Table 3, all four variables must be considered jointly. Survival analyses according to the immunological asset are reported in Fig. 4 and Table 4. To be noted and of particular interest, the rate of patient with a disease progression in the first 3 months of treatments was higher in the poor prognostic group (55% vs 11%), and the risk of relapse resulted in one-third of the others (HR = 0.28, CI 0.12–0.64, P = 0.003) identifying a potentially linked hyper progression, potentially related to the high value of Gr-MDSC and eosinophils and low value of NLR and neutrophils.
Discussion
Immune checkpoint inhibitors, such as the anti-PD-1 antibody nivolumab, have shown remarkable clinical activity in different types of cancer, including NSCLC. Although nivolumab has been shown to have high efficacy as a second-line treatment for NSCLC regardless of histological type (non-squamous or squamous) and PD-L1 status, a minority of patients still show rapid disease progression generally in the first 3 months of treatment. Therefore, more and better predictive/prognostic, dynamic biomarkers are urgently needed to differentiate patients who are most likely to benefit from treatment; delimit the most appropriate treatment timing, dosage, and schedule; and avoid ineffective, costly, and/or potentially toxic treatment of patients who will not benefit. In addition to the well-known biomarkers (e.g., PD-L1; TMB), preclinical and clinical investigations of pre- and post-treatment peripheral blood and immunological characteristics have been shown to be useful in predicting the success of checkpoint inhibitors for NSCLC [17], through the evaluation of different biological cells including neutrophil eosinophil [18, 19], myeloid CD33+CD11b+HLA-DR− [20] myeloid and monocyte CD14+CD16−HLA-DRhi [21]. PD-1 and PD-L1 can be upregulated to play a pivotal role in antitumor immunity not only by tumour and T cells but also by inflammatory, suppressor, regulatory and stromal cells such as MDSC, macrophages, Tregs and endothelial cells [22, 23]. In locally advanced and metastatic melanoma patients treated with PD-1 and CTLA4 checkpoint blockade, the rate of intratumoral PD-L1+ dendritic cells (DCs) and PD-L1+ macrophages positively correlated with complete clinical responses to treatment [22]. Along a similar line, the percentages of PD-L1+ DCs and PD-L1+ macrophages positively correlated with clinical responses to PD-1 blockade in ovarian cancer patients [24]. Taken together with our current data and several other preclinical data [24, 25], these findings support the crucial role of myeloid cells in antitumor immunity and in the clinical response to checkpoint inhibition. Interestingly, in our work, we also found higher baseline circulating PD-L1 levels in patients with ORR. These data suggest that not only the tumour-associated microenvironment but also the circulating levels of PD-L1 and immunosuppressive cells deserve to be investigated to predict the activity of checkpoint inhibitors. The emerging role of myeloid-derived suppressor cells (MDSC) in relationship with immune-checkpoint inhibitor therapy in solid tumours and in particular in non-small cell lung cancer is growing steadily. Indeed, MDSC plays a crucial role in cancer progression by suppressing the activity of various immune cells and promoting angiogenesis an metastasis. The MDSCs are acquiring a pivotal role as regards the resistance to ICI treatment as identified by our research, in which patients with baseline high value of Gr-MDSC (≥ 6 cell/μl) showed a significantly improved survival compared with those patients with a lower baseline data.
The most interesting finding of our research was the identification of a significant correlation between the immune asset (including high Gr-MDSC, low absolute neutrophil count, high eosinophil count, NLR < 3) and improvement in survival (PFS and OS).
These evidences confirmed the crucial role of the tumour microenvironment (TME) including very different immune suppressive cells such as Treg, MDSC and tumour-associated macrophages (TAM) which contribute to the resistance to ICI therapy [14].
Furthermore, the current approach and subsequent results enabled us to identify those patients with a poor prognosis under immunotherapy, which is not easily or clearly done with currently available biomarkers. However, it is important to note that P values calculated in the present analysis have not been adjusted for multiple comparisons. Therefore, findings of borderline significance should be interpreted with caution. Nonetheless, the results of the current and previous studies have important biological and clinical implications. Our findings confirm that preclinical levels of neutrophils and other myeloid cell subpopulations play a relevant role in the mechanisms of NSCLC clinical resistance to checkpoint inhibitors [26]. Along this line, some therapeutic strategies have been proposed to target the myeloid cell compartment to improve the clinical efficacy of checkpoint inhibitors [27]. In future, the Gr-MDSC immuno-asset defined in the present study should be included in larger clinical studies to confirm its performance in predicting clinical phenotype and outcomes40, as well as determine its use in shaping rational NSCLC patient selection and treatment strategies and defining the most appropriate sequential therapy [28]. Furthermore, a comparison of our immune-asset biomarker sequence with other biomarkers (e.g., circulating and tissue-based) may also be useful for selecting patients based on TMB or Teff gene expression.
Change history
20 August 2019
Acknowledgements section was missing
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- PD-L1:
-
Programmed cell death-ligand 1
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- TMB:
-
Tumour mutational burden
- Gr-MDSC:
-
Granulocytic myeloid-derived suppressor cells
- ORR:
-
Overall response rate
- NLR:
-
Neutrophil-to-lymphocyte ratio
- NK:
-
Natural killer
- ICI:
-
Immune checkpoint inhibitor
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
Lee CK, Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288–300.
Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704.
Dolan DE, Gupta S. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control. 2014;21(3):231–7.
Doroshow DB, Sanmamed MF, Hastings K, et al. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res. 2019. https://doi.org/10.1158/1078-0432.CCR-18-1538.
Thunnissena E, de Langenb AJ, Smitb EF. PD-L1 IHC in NSCLC with a global and methodological perspective. Lung Cancer. 2017;113:102–5.
Vogelstein B, Papadopoulos N, Velculescu VE. Cancer genome landscapes. Science. 2013;339:1546–58.
Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150(6):1121–34.
Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–8.
Peters S, Creelan B, Hellmann MD, et al. Abstract CT082: Impact of tumour mutation burden on the efficacy of the first-line nivolumab in stage iv or recurrent non-small cell lung cancer: an exploratory analysis of CheckMate 026. Cancer Res. 2017. https://doi.org/10.1158/1538-7445.AM2017-CT082.
Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage iv or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26.
Addeo A, Banna GL, Weiss GJ. Tumor mutation burden-from hopes to doubts. JAMA Oncol. 2019. https://doi.org/10.1001/jamaoncol.2019.0626.
Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;23(11):955–65.
Park SM, Youn JI. Role of myeloid-derived suppressor cells in immune checkpoint inhibitor therapy in cancer. Arch Pharm Res. 2019. https://doi.org/10.1007/s12272-019-01165-6.
Kowanetz M, Zou W, Mccleland M, et al. Pre-existing immunity measured by teff gene expression in tumor tissue is associated with atezolizumad efficacy in NSCLC. J Thor Oncol. 2017;12(11, Supplement 2):S1601–S2433 (abstract MA 05.09).
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–41.
Fridman WH, Zitvogel L, Sautès-Fridman C, et al. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–34.
Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2018;29(2):524.
Ferrucci PF, Gandini S, Cocorocchio E, et al. Baseline relative eosinophil count as a predictive biomarker for ipilimumab treatment in advanced melanoma. Oncotarget. 2017;8(45):79809–15.
Sade-Feldman M, Kanterman J, Klieger Y, et al. clinical significance of circulating CD33+CD11b+HLA-DR− myeloid cells in patients with stage IV melanoma treated with ipilimumab. Clin Cancer Res. 2016;23:5661–722.
Shin DS, Ribas A. The evolution of checkpoint blockade as cancer therapy: what’s here, what’s next? Curr Opin Immunol. 2015;33:23–35.
Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Rev Immunol. 2015;15(1):45–56.
Lin H, Wei S, Hurt EM, et al. Host expression of PD-L1 determines the efficacy of PD-L1 pathway blockade-mediated tumour regression. J Clin Invest. 2018;128(2):805–15.
Tang H, Liang Y, Anders RA, et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumour regression. J Clin Invest. 2018;128(2):580–8.
Glodde N, Bald T, van den Boorn-Konijnenberg D, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity. 2017;47(4):789–802.
De Henau O, Rausch M, Winkler D, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016;539:443–7.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AP and FdM served as advisor/consultant for Bristol-Myers Squibb outside the submitted work. The other authors declare no competing interests.
Ethical approval
This research was approved by the Institutional Ethics Committee and was carried out according to the Ethical Principles of the Declaration of Helsinki (3388B).
Informed consent
All patients provided written informed consent prior to study inclusion.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Passaro, A., Mancuso, P., Gandini, S. et al. Gr-MDSC-linked asset as a potential immune biomarker in pretreated NSCLC receiving nivolumab as second-line therapy. Clin Transl Oncol 22, 603–611 (2020). https://doi.org/10.1007/s12094-019-02166-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02166-z