Abstract
Purpose
To evaluate the prognostic factors associated with survival in patients treated with neoadjuvant treatment [chemoradiotherapy (CRT) or chemotherapy] followed by surgery (CRTS) in patients with stage IIIA-N2 non-small cell lung cancer (NSCLC).
Methods
A retrospective study was conducted of 118 patients diagnosed with stage T1-T3N2M0 NSCLC and treated with CRTS at 14 hospitals in Spain between January 2005 and December 2014. Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan–Meier method and compared using the log-rank test. Cox regression analysis was performed.
Results
Surgery consisted of lobectomy (74.5% of cases), pneumectomy (17.8%), or bilobectomy (7.6%). Neoadjuvant treatment was CRT in 62 patients (52.5%) and chemotherapy alone in 56 patients (47.5%). Median follow-up was 42.5 months (5–128 months). 5-year OS and PFS were 51.1% and 49.4%, respectively. The following variables were independently associated with worse OS and PFS: pneumonectomy (vs. lobectomy); advanced pathologic T stage (pT3 vs. pT0–pT2); and presence of persistent N2 disease (vs. ypN0-1) in the surgical specimen.
Conclusions
In this sample of patients with stage IIIA-N2 NSCLC treated with CRTS, 5-year survival (both OS and PFS) was approximately 50%. After CRTS, the patients with the best prognosis were those whose primary tumour and/or mediastinal nodal metastases were downstaged after induction therapy and those who underwent lobectomy. These findings provide further support for neoadjuvant therapy followed by surgery in selected patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients diagnosed with stage IIIA-N2 non-small cell lung carcinoma (NSCLC) comprise a heterogeneous group. Treatment outcomes in patients treated with surgery or radiotherapy alone are poor, with 5-year overall survival (OS) rates of < 10% [1]. The current standard of care, based on the findings from two meta-analyses [2, 3], is concomitant chemoradiotherapy (CRT), which achieves better but still relatively modest survival outcomes (5-year OS of approximately 20%) [2, 3]. Given these poor outcomes, numerous phase II and III trials have evaluated neoadjuvant treatment followed by surgery in patients with potentially resectable N2 disease [4,5,6,7]. Although no randomized study has demonstrated a survival advantage for this approach versus CRT alone, the results of these trials suggest that a subgroup of patients could benefit—in terms of local control and survival—from the addition of surgery [4, 8, 9].
Compared to neoadjuvant chemotherapy alone, CRT improves downstaging of primary tumour and lymph nodes, and also increases complete pathological response rates. However, surprisingly, these improvements have not resulted in better OS [8, 10]. In this context, the aim of this retrospective Spanish multicentre study was to evaluate the prognostic factors associated with survival in patients treated with neoadjuvant treatment [chemoradiotherapy (CRT) or chemotherapy] followed by surgery (CRTS) in patients with stage IIIA-N2 non-small cell lung cancer (NSCLC).
Materials and methods
Study population
This retrospective study was based on clinical records from 14 hospitals in Spain. The study was approved by the ethics committees of all participating hospitals. The study was supported by the Radiation Oncology Clinical Research Group (GICOR) and the GOECP-SEOR (Oncologic Group for the Study of Lung Cancer-Spanish Society of Radiation Oncology).
The study sample included patients diagnosed between the years 2005 and 2014 with potentially resectable stage IIIA-N2 NSCLC (T1-3 N2 M0) and treated with radical intent induction therapy followed by surgery. Exclusion criteria were: stage cT4 disease and definitive chemoradiotherapy (dCRT). Inclusion criteria were: mediastinal lymph node involvement on imaging studies. Whenever possible, the mediastinal disease was confirmed histologically by endobronchial ultrasound (EBUS), endoscopic ultrasound (EUS), or mediastinoscopy and treated with chemotherapy or CRT followed by surgery. Clinical staging was based on thoracic computed tomography (CT), positron-emission tomography-CT (PET-CT), and brain CT or magnetic resonance imaging (MRI) and classified according to the TNM staging system (seventh edition).
Treatment scheme and follow-up
Neoadjuvant treatment consisted of three to four cycles of chemotherapy alone (platinum-based doublets) or concomitant CRT. The radiotherapy dose ranged from 45 to 66 Gy and was delivered with a conventional fractionation scheme (1.8–2 Gy/fraction) to the tumour volume. No elective nodal irradiation was performed. The radiotherapy technique was three-dimensional radiotherapy (3D-RT).
Response to neoadjuvant treatment was evaluated using the Response Evaluation Criteria in Solid Tumours (RECIST) based on either CT or PET-CT. In some cases—depending on the specific protocol at the participating hospital—the mediastinal nodes were reassessed histologically by EUS, EBUS, or mediastinoscopy after induction therapy. The time elapsed from finalization of neoadjuvant treatment until surgery was recorded in days. Surgical treatment consisted of lobectomy or pneumonectomy, with homolateral hilar and mediastinal lymphadenectomy.
Pathologic complete response (pCR) was defined as the absence of viable tumour in the resected specimen. Delivered adjuvant treatment depended on the specific protocol of each centre. Follow-up was performed weekly during neoadjuvant treatment, postoperatively every 3–6 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Acute and chronic haematological, gastrointestinal, thoracic, and pulmonary toxicity were recorded according to the Common Toxicity Criteria for Adverse Events (v. 4.0).
The date of recurrence was defined as the day an abnormal imaging test result was detected during follow-up. Locoregional relapse was defined as a recurrence to the ipsilateral lung and/or nodal regions (hilum, mediastinum, supraclavicular fossa). Distant relapse was defined as a recurrence in other locations according to the staging criteria of the American Joint Committee on Cancer (AJCC).
Statistical analysis
OS was defined as the time elapsed from the date of pathological diagnosis until the date of death or last follow-up. PFS was calculated from the date of the pathological diagnosis until first recurrence, death from any cause, or last final follow-up. OS and PFS were estimated using the Kaplan–Meier method. The log-rank test was used to compare survival curves between groups. Multivariate analysis was performed using the Cox proportional hazards model to determine the variables significantly associated with survival. Statistical significance was set at p < 0.05. Statistical analyses were performed using SPSS statistical software, version 22.0 (SPSS statistics for windows, IBM Corporation, Armonk, NY).
Results
Patient characteristics
The initial database search identified 294 patients diagnosed with stage IIIA-N2 NSCLC. Of these, 176 patients had received dCRT and were excluded from the study. A total of 118 patients were included. Patient characteristics are described in Table 1. In 69 cases (58.5%), mediastinal involvement was confirmed histologically at diagnosis. Median age was 62 years (range 41–78) and 94 patients (79.7%) were men. The Eastern Cooperative Oncology Group (ECOG) score was 0 or 1 in nearly all cases (99.2%). In most cases (72 patients; 61%), only a single mediastinal lymph node station was involved at diagnosis. The clinical T stage was as follows: cT1 in 30 patients (25.5%), cT2 in 41 patients (35%), and cT3 in 46 patients (39.3%). Histologically, adenocarcinoma accounted for just over half of the cases (50.4%).
Neoadjuvant and postoperative treatment
Neoadjuvant treatment consisted of CRT in 66 patients (56%) and chemotherapy alone in 52 patients (44%). Neoadjuvant radiotherapy doses were over 50 Gy in 47 patients (71.2%). The most common fractionation schedule (69.7%) was 2 Gy/session. Radiotherapy was delivered using the 3D technique in 97% of patients who underwent radiotherapy. In most cases (93.2%), the chemotherapy regimen consisted of three cycles with a platinum-based doublet. Postoperatively, 51 patients received adjuvant treatment: 20 (39.2%) received only adjuvant chemotherapy, 20 (39.2%) underwent only radiotherapy (45–50 Gy). and 11 (21.6%) received both treatments.
Neoadjuvant and postoperative therapy were well tolerated. Toxicity ≥ grade three was uncommon. Grade ≥ 3 haematological toxicity was observed only in two patients (1.9%) (one in the neoadjuvant chemotherapy group and one in the neoadjuvant CRT group) and grade ≥ 3 esophagitis toxicity was observed in four patients (3.6%) (one in the neoadjuvant chemotherapy group and three in the CRT group). No other severe toxicities were observed.
Surgery
Response to neoadjuvant treatment was assessed by PET-CT in 55 patients (46.6%) and by CT in 63 patients (53.4%). Most patients had partial radiological response to neoadjuvant treatment (86.4%), with 11 patients (9.3%) achieving a complete response. The mediastinal nodes were histologically re-evaluated prior to surgery by EBUS and/or mediastinoscopy in 47 patients (39.8%), with negative findings in most cases (89.3%). The median time interval from completion of neoadjuvant therapy until surgery was 60 days (Table 2).
The most frequent surgical procedure was lobectomy (74.6%), followed by pneumectomy (17.8%) and bilobectomy (7.6%). Two deaths (4%), both treated with neoadjuvant CRT, due to acute respiratory distress syndrome occurred within 90 days of surgery; of these, one patient had undergone lobectomy and the other pneumectomy.
Pathologic findings
A complete resection (R0) was achieved in most cases (95,8%) while three patients (2.6%) presented microscopic involvement (R1) of the margins and one patient (0.9%) had macroscopic involvement (R2). The pathological T stage in the sample was distributed as follows: ypT0 in 34 patients (28.8%), ypT1 in 49 (41.5%), ypT2 in 22 (18.6%), and ypT3 in 13 (11%). The pathological N stage was ypN0 in 81 patients (68.6%), ypN1 in 7 (5.9%), and ypN2 in 30 (25.4%). pCR in the primary tumour and mediastinal nodes (pT0pN0) was obtained in 32 patients (27.1%), 6 (18.7%) treated with CHT chemotherapy neoadjuvant vs. 26 (81.2%) treated with CRT neoadjuvant (p < 0.001). A downstaging from N2 to ypN1-N0 was observed in 88 patients (74.6%), 33 (37.5%) treated with CHT chemotherapy neoadjuvant vs. 55 (62.5%) treated with CRT neoadjuvant (p < 0.001).
Overall survival
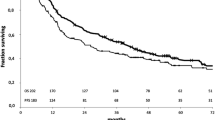
The median follow-up time was 42.5 months, with a mortality rate of 43.2% (51 patients). Median OS was 61 months (95% confidence interval [CI], 39.9–82.1). 5-year OS was 51.1% (95% CI, 40.1–62.0). By pathological T stage, 5-year OS rates were: ypT0 (65.3%), ypT1 (55%), ypT2 (40.4%), and ypT3 (23.1%) (p < 0.001, Fig. 1). Patients who experienced nodal downstaging presented a significantly better 5-year OS than patients with persistent N2 disease (59.2% vs. 28.5%; p < 0.001, Fig. 1). The median OS in the lobectomy group was significantly higher than in the pneumonectomy group (82 vs. 35 months; p = 0.012), with a significant difference in 5-year OS (54.3% vs. 36.6%; p = 0.06). 5-year OS rates were significantly better in patients who achieved pCR versus those with persistent tumour disease (65.4% vs. 46.3%, p = 0.03), although differences in median OS were not significant (82 vs. 56 months, p = 0.12) (Fig. 1).
Based on the univariate and the Cox multivariate analyses, the following variables were independently associated with worse OS: advanced pathologic T stage (pT3 vs. pT0–pT1), pneumonectomy (vs. lobectomy), and persistent N2 (vs. ypN0-1). The type of neoadjuvant treatment (CRT vs. chemotherapy) was not a prognostic factor associated with OS (Table 3).
Progression-free survival
During follow-up, 55 patients (46.6%) experienced a recurrence. The median time to first recurrence was 32 months. Locoregional recurrence occurred in 29 patients (24.6%), distant in 39 (33.1%), and both in 13 patients (11%).
The median PFS was 58 months (95% CI, 22.6–93.4) with a 5-year PFS of 49.4% (95% CI, 39.0–59.7). By pathological T stage, 5-year PFS rates were: ypT0 (65.4%), ypT1 (55.1%), ypT2 (32.7%), and ypT3 (15.4%) (p < 0.001, Fig. 2). PFS was better in patients who achieved nodal downstaging compared with persistent N2 (60.3% vs. 18.4%, p < 0.001). Median PFS was significantly longer in patients who underwent lobectomy versus pneumonectomy (61 vs. 32 months, p = 0.008), with a non-significant difference in 5-year PFS in the lobectomy group (51.8% vs. 38.6%, p = 0.08). Patients who achieved pCR presented a better 5-year PFS than those with persistent disease (68.7% vs. 42.4%), but this difference did not reach statistical significance (p = 0.05).
Based on the univariate and Cox multivariate analyses, advanced pathologic T stage, pneumonectomy, and persistent N2 were independently associated with worse PFS. The type of neoadjuvant treatment (CRT vs. chemotherapy) was not an independent prognostic factor associated with PFS (Tables 3, 4).
Discussion
The aim of this study was to evaluate treatment outcomes in a large sample of patients with stage IIIA-N2 NSCLC who received neoadjuvant CRT or chemotherapy followed by surgery. We also sought to determine predictors of survival. To our knowledge, this is the largest multicentre Spanish study to date to specifically evaluate treatment outcomes in this population of lung cancer patients—that is, patients diagnosed with stage IIIA-N2 NSCLC treated with CRT or chemotherapy followed by lobectomy or pneumonectomy.
Previous studies that have evaluated a similar treatment strategy (neoadjuvant therapy followed by surgery) have reported 5-year OS rates from 14% to 48%, with a median OS of 16–59 months [4, 5, 7,8,9,10,11]. In this context, the results of our series—5-year OS of 51.1% and median OS of 61 months—are promising. However, it should be emphasized that we included only patients who completed both neoadjuvant treatment and surgery. This is relevant because previous studies have shown lower survival rates in patients who do not undergo surgery as planned after neoadjuvant therapy [12, 13]. For example, Kim et al. [12] evaluated 664 patients with stage N1 NSCLC, 574 of whom completed the planned surgery. In that series, 5-year OS was worse in the patients who did not undergo surgery compared to those treated surgically (15% vs. 48%, p < 0.0001). Nonetheless, despite the selection bias in our study, we believe that our findings could potentially be extrapolated to other hospitals and regions due to the multicentric study design.
A substantial proportion (41.5%) of the patients in our sample did not undergo invasive mediastinal staging at any point. In Spain, the usual treatment for patients with N2 disease is neoadjuvant therapy followed by surgery. However, if the status of the mediastinal nodes is not confirmed histologically after neoadjuvant therapy, there is a risk of overdiagnosis as some patients may be diagnosed incorrectly with persistent N2 disease [14] or underdiagnosis (e.g. occult N3 disease) [14], which could ultimately affect the treatment decision and patient survival. That said, the presence or absence of histological confirmation was not a predictor of survival in our study.
There is some controversy regarding the value of surgery in patients with multi-station N2 disease and/or bulky disease (≥ 3 cm) [15]. In our study, multiple nodal stations were involved in 39% of cases while 6.8% of patients had bulky disease; however, neither of these two factors was associated with worse survival, a finding that suggests that surgery may be feasible in these patients.
The optimal neoadjuvant scheme remains unclear [15]. This controversy is reflected in the neoadjuvant treatment administered in our multicentric study, which was divided more or less evenly between CRT (55.9%) and chemotherapy alone (44.1%). Interestingly, the specific neoadjuvant therapy scheme, including high-dose induction CRT, was not predictive of survival on the univariate analysis, perhaps because most of the patients treated with chemotherapy alone received postoperative radiotherapy. Although our study included patients treated with both low (45–50 Gy) and high doses (60–66 Gy) of radiotherapy, a randomized trial is necessary to clarify whether the efficacy of high-dose (60 Gy at 2 Gy/session) CRT is comparable to chemotherapy alone.
The value of performing invasive mediastinal staging after induction therapy prior to surgery is also controversial [15]. Yang et al. evaluated 111 patients with stage IIIA pN2 NSCLC who underwent induction therapy followed by lobectomy, finding that pathologic mediastinal restaging allows clinicians to select those patients most likely to benefit from surgery; in that study, mediastinal restaging vs. no restaging was associated with better 5-year OS (45.2% vs. 13.9%, p = 0.004). Postoperative downstaging of the mediastinal nodes based on the surgical specimen was associated with even better 5-year OS rates (59.2% vs. 28.5%, p < 0.001) [16]. In our study, pathologic mediastinal restaging following induction therapy was not a predictor of survival, perhaps due to the delayed response to neoadjuvant radiotherapy, which could explain why early mediastinal restaging after induction therapy may not correlate closely with the mediastinal downstaging observed in the surgical specimen.
We found no association between survival and the time interval from induction therapy to surgery. This finding contrasts with the results of a recent study [17] in which patients who underwent surgery > 6 weeks after induction therapy had a worse OS, suggesting that 90-day postoperative mortality rates may increase due to radiation pneumonitis or fibrotic changes. Nonetheless, even though nearly 75% of patients in our sample underwent surgery more than 6 weeks after completing induction therapy, our 5-year OS rates are comparable to the best outcomes reported to date. Nonetheless, it should be noted that a large percentage of our sample (40%) received chemotherapy alone (rather than CRT), which could explain the reduced postoperative mortality associated with radiation pneumonitis.
With regard to the impact of the surgical procedure (pneumectomy vs. lobectomy) on treatment outcomes, we found no association between postoperative morbidity or mortality in patients who underwent pneumonectomy, probably due to careful patient selection (young age, good general and functional status). By contrast—consistent with the findings reported by Marulli et al. [18]—median OS was worse in the patients in our series who underwent pneumectomy compared to lobectomy (35 vs. 82 months). For this reason, as suggested by the findings of the Intergroup 0139 trial [4], the most appropriate treatment in these patients is probably dCRT.
Study strengths and limitations
The main limitation of the present study is its retrospective design, which could have led to a selection bias. In addition, we included only patients who underwent surgery after neoadjuvant therapy, and therefore the results cannot be generalized to all patients with stage IIIA-N2 NSCLC given that some patients never reach the surgical phase. Another potential limitation is the heterogeneity of the sample, particularly the presence or lack of post-induction histological confirmation of the mediastinal nodes and differences among the patients in the specific neoadjuvant regime. However, a comprehensive regression analysis was performed to account for differences in those two variables. Although the heterogeneity in our sample could be considered a limitation, it is important to note that this variability in patient characteristics reflects the complex and diverse nature of managing this patient population in routine clinical practice.
Conclusions
In this retrospective, multi-institutional study involving patients with stage IIIA-N2 NSCLC, neoadjuvant treatment followed by surgery achieved excellent long-term survival outcomes with acceptable morbidity. Our findings suggest that the patients most likely to benefit from multimodal treatment are: patients who undergo lobectomy (rather than pneumectomy), and whose primary tumour and/or mediastinal lymph nodes are downstaged after induction therapy. These findings provide further support for the use of neoadjuvant therapy followed by surgery in selected patients.
References
Martini, et al. The role of surgery in N2 lung cancer. Surg Clin North Am. 1987;67:1037–49.
Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90.
O’Rourke N, Roqué I Figuls M, Farré Bernadó N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010. https://doi.org/10.1002/14651858.CD002140.pub3.
Albain KS, Swann RS, Rusch VW, Turrisi AT 3rd, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374(9687):379–86.
Pless M, Stupp R, Ris H, Stahel RA, Weder W, Thierstein S, et al. Final results of the SAKK 16/00 trial: A randomized phase III trial comparing neoadjuvant chemoradiation to chemotherapy alone in stage IIIA/N2 non-small-cell lung cancer (NSCLC). Ann Oncol. 2014;25(suppl 4):iv417.
Katakami N, Tada H, Mitsudomi T, Kudoh S, Senba H, Matsui K, et al. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer. 2012;118(24):6126–35.
Eberhardt WEE, Pöttgen C, Gauler TC, Friedel G, Veit S, Heinrich V, et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE). J Clin Oncol. 2015;33(35):4194–201.
Aggarwal C, Li L, Borghaei H, Mehra R, Somaiah N, Turaka A, et al. Multidisciplinary therapy of stage IIIA non-small-cell lung cancer: long-term outcome of chemoradiation with or without surgery. Cancer Control. 2014;21(1):57–62.
Darling GE, Li F, Patsios D, Massey C, Wallis AG, Coate L, et al. Neoadjuvant chemoradiation and surgery improves survival outcomes compared with definitive chemoradiation in the treatment of stage IIIA N2 non-small-cell lung cancer. Eur J Cardio-Thoracic Surg. 2015;48(5):684–90.
Xu X-L, Dan L, Chen W, Zhu S-M, Mao W-M. Neoadjuvant chemoradiotherapy or chemotherapy followed by surgery is superior to that followed by definitive chemoradiation or radiotherapy in stage IIIA (N2) non small-cell lung cancer: a meta-analysis and system review. Onco Targets Ther. 2016;9:845–53.
van Meerbeeck JP, Kramer GW, Van Schil PE, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. J Natl Cancer Inst. 2007;99:442–50.
Kim HK, Cho JH, Choi YS, Zo JI, Shim YM, Park K, et al. Outcomes of neoadjuvant concurrent chemoradiotherapy followed by surgery for non-small-cell lung cancer with N2 disease. Lung Cancer. 2016;96:56–62.
Cerfolio RJ, Maniscalco L, Bryant AS. The treatment of patients with stage IIIA non-small cell lung cancer from N2 disease: who returns to the surgical arena and who survives. Ann Thorac Surg. 2008;86(3):912–20.
Rocco G, Nason K, Brunelli A, Varela G, Waddell T, Jones DR. Management of stage IIIA (N2) non-small-cell lung cancer: a transatlantic perspective. Eur J Cardiothorac Surg. 2016;49(4):1025–7.
Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-small cell lung cancer, version 5. 2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–35.
Yang CF, Adil SM, Anderson KL, Meyerhoff RR, Turley RS, Hartwig MG, et al. Impact of patient selection and treatment strategies on outcomes after lobectomy for biopsy-proven stage IIIA pN2 non-small cell lung cancer. Eur J Cardiothorac Surg. 2016;49(6):1607–13.
Gao SJ, Corso CD, Wang EH, Blasberg JD, Detterbeck FC, Boffa DJ, et al. Timing of surgery after neoadjuvant chemoradiation in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12(2):314–22.
Marulli G, Verderi E, Zuin A, Schiavon M, Battistella L, Perissinotto E, et al. Outcomes and prognostic factors of non-small-cell lung cancer with lymph node involvement treated with induction treatment and surgical resection. Interact Cardiovasc Thorac Surg. 2014;19(2):256–62.
Acknowledgements
The authors wish to thank David Saldaña, Thoracic Surgery of Hospital Universitario Ramón y Cajal, Madrid; Ignacio Muguruza Trueba, Thoracic Surgery of Hospital Universitario Rey Juan Carlos, Móstoles; Pedro López Castro, Thoracic Surgery of Hospital Germans Trias i Pujol, Badalona and Rafael Aguilo Espases, Thoracic Surgery of Hospital del Mar, Barcelona, for the collaboration in this study. The author wish to thank Bradley Londres for translating and editing this manuscript. The study was supported by the Radiation Oncology Clinical Research Group (GICOR) and the GOECP-SEOR (Oncologic Group for the Study of Lung Cancer-Spanish Society of Radiation Oncology).
Funding
The translation of this work was supported financially by the Spanish Society of Radiation Oncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The present study involved human participants, and it was conducted considering ethic responsibilities according to the World Medical Association and the Declaration of Helsinki.
Informed consent
The study was approved by the ethics committees of all participating hospitals. Informed consent statement was not necessary for retrospective study. Patients provided informed consent for treatment as per standard procedure at the individual institutions.
Rights and permissions
About this article
Cite this article
Couñago, F., Montemuiño, S., Martin, M. et al. Prognostic factors in neoadjuvant treatment followed by surgery in stage IIIA-N2 non-small cell lung cancer: a multi-institutional study by the Oncologic Group for the Study of Lung Cancer (Spanish Radiation Oncology Society). Clin Transl Oncol 21, 735–744 (2019). https://doi.org/10.1007/s12094-018-1976-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1976-3