Abstract
Background
To investigate the efficacy and toxicity of 68Ga-PSMA-HBED-CC (68Ga-PSMA) PET-CT-guided RT in the treatment of oligometastatic prostate cancer retrospectively.
Methods
A total of 23 prostate cancer patients with biochemical relapse, of which 13 were castration sensitive (CS) and 10 castration resistant (CR), were treated with intensity-modulated and image-guided RT (IMRT-IGRT) on ≤3 metastases detected by 68Ga PSMA PET-CT. Androgen deprivation therapy was continued in CR patients.
Results
A total of 38 metastases were treated. The involved sites were pelvic bone (n = 16), pelvic lymph nodes (n = 11), paraaortic lymph nodes (n = 6), ribs (n = 3) and vertebral body (n = 2). The median PSA prior to RT was 1.1 ng/mL (range 0.1–29.0 ng/mL). A median dose of 43.5 Gy (range 30–64 Gy) was delivered by IMRT-IGRT in 12–27 fractions. At a median follow-up of 7 months (range 2–17 months), 19 patients (83%) were in remission. Four patients (17%) developed distant recurrences. The actuarial 1-year LC, PFS and OS rates were 100, 51 (95% CI 8–83%) and 100%. Univariate analysis demonstrated a statistically significantly better PFS in CS patients as compared to CR patients (1-year PFS 67 vs. 0%, p < 0.01). One patient experienced grade 2 acute gastrointestinal toxicity. Grade 3 or more toxicity events were not observed.
Conclusions
By providing optimal LC, low toxicity and a promising PFS in CS patients, the current retrospective study illustrated that 68Ga PSMA PET-CT-guided RT may be an attractive treatment strategy in patients with oligometastatic prostate cancer. Validation by randomized trials is eagerly awaited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PC) is the most common tumor in elderly males worldwide [1]. Androgen deprivation therapy (ADT) with luteinizing hormone-releasing hormone (LHRH) analog remains the cornerstone in the treatment of metastatic PC by delaying progression and improving overall survival (OS). Unfortunately, the majority of these patients develop within a median time of 18–24 months castration-resistant prostate cancer (CRPC), which remains an incurable disease despite advances in systemic treatment [2]. The role of radiotherapy (RT) in metastatic PC patients is typically restricted as palliative treatment of symptomatic bone metastases. However, PC patients with limited metastatic disease, so-called oligometastases, have a much better prognosis than patients with extensive metastatic disease, as the oligometastatic disease state is considered to display a limited metastatic capacity and less aggressive behavior [3,4,5]. RT may have a potential role in the treatment of oligometastatic PC by delaying disease progression and postponing systemic treatment [6]. The implementation of stereotactic body RT (SBRT) is an effective and safe tool in the eradication of oligometastases in PC patients: local control rates up to 100% with no grade 3 toxicity [7,8,9]. These investigators determined the oligometastatic status of their patient according to 11C-choline positron emission tomography computed tomography (CT) (11C-choline-PET/CT), 18-fluorodeoxyglucose (FDG) PET/CT, magnetic resonance imaging (MRI) or bone scan [10]. Berkovic et al. [8] reported median time to clinical progression of 18 months (range 6–23 months), while Ahmed et al. [7] reported 6- and 12-month estimates of cause-specific survival (CSS) as 100%.
The challenge, however, in those patients is the early detection of oligometastases, since conventional imaging modalities, such as CT and bone scan, are inaccurate in the detection of small metastases. Conventional 18-FDG PET has been widely used for various tumors [11,12,13]; its role in PC is however limited due to highly variable FDG-avidity of PC. On the contrary, 11C-choline-PET is considered as a useful tool for the assessment of recurrent disease with detection rates between 21 and 82% [14], especially in case of PSA levels >2 ng/mL [15].
Recently, there is an increasing investigation about specific markers related to PC. Prostate-specific membrane antigen (PSMA), a type II trans-membrane protein, is a well-characterized imaging biomarker of which the expression has been shown to increase from benign to prostatic hyperplasia to prostatic adenocarcinoma [16]. Many trials also demonstrated the accuracy of 68Ga-PSMA PET/CT in the identification of both local and distant recurrences at low levels of PSA, with a detection rate for recurrent disease of approximately 85–90% [17,18,19]. Particularly in the case of recurrent PC with lymph node metastases, PET using 68Ga-PSMA has shown a higher detection rate compared to 11C-choline-PET [20]. Clinical data on the implementation of 68Ga-PSMA PET/CT-guided RT in oligometastatic PC are scarce. The purpose of this retrospective study is to assess the efficacy and toxicity of 68Ga-PSMA PET/CT-guided RT in the treatment of oligometastatic PC.
Materials and methods
Patients
The study population consisted of 23 patients with biopsy-proven PC initially treated with radical prostatectomy and/or RT and/or androgen deprivation therapy (ADT) between January 2003 and May 2015. All patients presented with biochemical relapse, of which 13 were castration sensitive (CS) and 10 castration resistant (CR), and were diagnosed with oligometastatic disease, defined as one to three lesions, by 68Ga-PSMA PET/CT. The treatment decision for RT was made after multidisciplinary oncology consultation. Ten patients (43%) were considered to be CR as they displayed three consecutive rises of PSA, 1 week apart, resulting in two 50% increases over the nadir with PSA >2.0 ng/mL and castrate serum levels of testosterone (<50 ng/dL or <1.7 nmol/L) [9] while being under treatment with LHRH analogs. All patients did also have subcutaneous luteinizing hormone-releasing hormone (LHRH) agonist and oral bicalutamide throughout treatment.
Patients were enrolled from two institutes for this retrospective analysis. This retrospective study complied with the regulations of the local institutional review board and the principles of the Declaration of Helsinki.
68Ga-PSMA PET/CT imaging
68Ga-PSMA PET/CT was started 60 min after intravenous administration of 140 ± 25 MBq 68Ga-PSMA (68Ga-PSMA-HBED-CC). A diagnostic CT (non-contrast enhanced) and PET data covering “mid-thigh—base of the skull” were acquired using state-of-the-art PET/CT systems. The effective dose associated with this procedure was 15.1 mSv (CT 12.1 mSv, PET 3.0 mSv). PET images were reconstructed using the vendor’s standard iterative reconstruction algorithms and the data were corrected for attenuation, scatter and random coincidences. PET/CT images were evaluated by experienced imaging physicians, and foci with increased 68Ga-PSMA accumulation were categorized as malignant/benign based on the integrated findings of signal intensity of PET and precise localization/corresponding structures on CT. The uptake intensity of 68Ga-PSMA in lesions was assessed semi-quantitatively by calculating the standardized uptake value (SUV). The pixel with the highest SUV value (SUVmax) within the lesion was used to represent the lesion intensity. In case of equivocal situations, additional imaging studies, such as MRI, were performed to distinguish metastasis from benign alterations.
Radiotherapy
All the treatments were performed in the supine position. Patients with pelvic bone or lymph node metastasis underwent 3-mm slice thickness CT with a comfortably full bladder and empty rectum [21]. Patients with paraaortic metastasis received a planning CT of the abdomen with 3-mm thickness. The CT scan of the entire chest with arms above the head in acquisition with wing-board was carried out for patients with rib metastases. Co-registration of the PSMA PET-CT and planning CT was carried out with MIM (Accuray, USA). An automated image registration software was used to perform CT- and PET-CT-based treatment planning for each patient, and registration was checked manually.
The gross tumor volume (GTV) included the visible gross tumor mass on CT, fully encompassing the PSMAPET positive volume. In case of lymph node metastases, no clinical target volume (CTV) margin was applied. The whole vertebra was delineated as CTV in case of vertebral bone metastases. For bone metastases outside the vertebra, a 5-mm margin was used to create the CTV. The GTV/CTV was expanded by 5 mm for all directions to create the planning target volume (PTV).
Bladder, rectum, sigmoid colon, small bowel, kidneys and spinal cord constituted the organs at risk (OAR) in the pelvic/paraaortic irradiation. Also, the lung, heart and esophagus were OAR during rib irradiation. The planning goals were to deliver at least 95% of the prescribed dose to at least 95% of the PTVs, while keeping the maximum dose below 105%. The dose volume constraints were derived from the ‘Quantitative Analyses of Normal Tissue Effects in the Clinic’ (QUANTEC) data [22].
All patients were treated by intensity-modulated and image-guided RT (IMRT-IGRT) once daily excluding weekends. A median dose of 45 Gy (range 40–52.5 Gy) was delivered in 15 fractions to 14 patients (61%) (Fig. 1). The other patients received 30 Gy in 12 fractions (n = 4), 64 Gy in 27 fractions (n = 3), 40 Gy in 20 fractions (n = 1) or 39 Gy in 13 fractions (n = 1) (Table 1). All the treatments were delivered using the TomoTherapy Hi-Art II System (Accuray, USA) or Axesse (Elekta AB, Stockholm, Sweden). Kilovoltage or megavoltage cone-beam CT was performed before each treatment session. An image fusion between the reference image and the cone-beam CT images before each treatment sessions was performed according to the bony anatomy and soft tissue in bone metastases and lymph node metastases, respectively.
a Pre-treatment with prostate-specific membrane antigen (PSMA) positron emission computed tomography (PET-CT) of a prostate cancer patient with a solitary retroperitoneal lymph node metastasis. b Planning CT with the superimposed radiation dose distribution and dose levels. The gross tumor volume (GTV) is indicated in blue, and the planning target volume (PTV) in red. A dose of 45 Gy was delivered in 15 fractions of 3 Gy
Clinical follow-up
Weekly clinical examination was performed during RT. Follow-up of patients was performed with 3 months interval. Toxicity was evaluated and scored according to the National Cancer Institute Common Toxicity Criteria (CTCAE) version 4.0. PSA was routinely assessed in follow-up visits. In case of an increase in PSA levels (at least 2 consecutive rises of PSA, 1 month apart or any PSA value over the nadir with PSA >2.0 ng/mL) during follow-up, imaging studies were performed.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (SPSS for Window, IBM Corp., Armonk, NY, USA). Actuarial LC, progression-free survival (PFS) and overall survival (OS) rates were estimated by Kaplan–Meier analysis. The time to event was calculated as the time interval from the date of diagnosis to the first clinical or radiological finding suggesting the recurrence of disease. Log-rank testing was used to evaluate the association between patient-related factors and treatment outcome. Correlations between parameters were calculated using the Pearson test. A p value of ≤0.05 was considered statistically significant.
Results
Patient characteristics
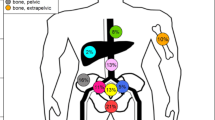
Patient characteristics are summarized in Table 1. The majority of the patients (n = 17) presented initially high-risk disease according to D’Amico classification system for PC. The median age was 68 years (range 54–78 years). The median pre-treatment PSA (before 68Ga-PSMA PET/CT imaging) was 1.1 ng/mL (range 0.1–29.0 ng/mL). Thirteen patients (56%) had solitary metastasis, five patients (22%) had two and five patients had three metastases. Among the entire cohort, three patients (23%) had synchronous bone and lymph node metastasis. Twenty patients (87%) had either bone (n = 10) or lymph node (n = 10) recurrences.
A total of 38 metastases were treated. The involved sites were pelvic bone (n = 16), pelvic lymph nodes (n = 11), paraaortic lymph nodes (n = 6), ribs (n = 3) and vertebral body (n = 2). None of the patients had local recurrence in the prostate. Median SUVmax of the oligometastatic disease was 11.3 (range 3.2–39.9). The PSA value at the time of the PET/CT was statistically significantly correlated with SUVmax of the metastatic lesion (r 0.771, p < 0.001).
There was a statistically significant difference in SUVmax between CS (median 7.6, range 3.2–24.8) and CR (median 16.4, range 3.7–39.9) patients (p = 0.03) (Fig. 2). The median PSA at the time of metastasis for CS and CR patients was 0.6 ng/mL (range 0.1–18.9 ng/mL) and 5.5 ng/mL (range 0.6–29.0 ng/mL), respectively (p = 0.04).
Patient outcome
The median follow-up of the entire cohort was 7 months (range 2–17 months). All patients were alive and no local recurrences at the irradiation site were observed at the last visit. Four patients developed recurrences outside the RT portal. Two patients with CS disease received androgen deprivation therapy together with abiraterone acetate, and two patients with CR disease were referred for systemic treatment with docetaxel.
The PFS rate was 100% at 6 months and 51% at 12 months (95% CI 8–83%). LC and OS were 100% at 1 year. Patients with CS oligometastatic disease displayed a statistically significantly superior PFS as compared to CR oligometastatic patients on univariate analysis (1-year PFS 67 vs 0%, p = 0.009) (Fig. 3).
The median PSA at the time of metastasis and at the last follow-up was 1.06 ng/mL (range 0.10–29.00 ng/mL) and 0.78 ng/mL (range 0.02–19.00 ng/mL), respectively (p = 0.08). Seventeen patients displayed a decrease in PSA levels compared to baseline and 6 patients presented with rising PSA during follow-up, of which 4 were with a documented recurrence outside the RT field as reported above.
Toxicity
There were no grade 3 or more acute or late toxicity in the study cohort according to CTCAE v4.0. One patient developed grade 2 acute diarrhea, and five patients had either grade 1 gastrointestinal and/or genitourinary toxicity.
Discussion
In the present retrospective study, we investigated the potential role of 68Ga PSMA PET/CT-guided RT in oligometastatic PC. Early detection of metastatic disease at low levels of PSA is essential in oligometastatic PC patients. The advantage of 68Ga PSMA PET/CT over other nuclear imaging modalities is that a PSA of 0.83 ng/mL was shown to be an effective cutoff value for recurrent PC [23]. In accordance, the median PSA at the time of the 68Ga PSMA PET/CT was 1.06 ng/mL in our patient population, although CR patients presented with a higher PSA than CS patients at the time of PSMA PET/CT imaging.
The rationale of the implementation of RT to 68Ga PSMA-avid oligometastases is to decrease the androgen-independent tumor burden. Hence, it might delay disease progression in CS patients and postpone further systemic therapy (including chemotherapy) in CR patients. Animal studies support the use of local treatment to eradicate androgen-independent clones [24, 25]. Preclinical data also suggest that the levels of PSMA are directly correlated to androgen independence and metastatic progression [10, 26]. These in vitro data support the clinical use of PC imaging targeting PSMA. Noticeable in the current study, 1-year PFS rates were 67 vs. 0% for patients with CS and CR oligometastatic disease, respectively (p = 0.009). This statistically significant difference in favor of CS may reflect the less aggressive behavior of oligometastatic hormone-sensitive disease as compared to more aggressive castration-resistant oligometastases. Those preliminary data need to be validated with large prospective data to optimize patient selection for metastases-directed therapies.
The impact of 68Ga PSMA PET/CT on decision-making in RT has been investigated by several authors [19, 27, 28]. Shakespeare et al. [28] reported the predictive role of 68Ga PSMA PET/CT in 54 PC patients treated with RT. In 46.3% of the patients, 68Ga PSMA PET/CT demonstrated evidence of disease after negative conventional images. They also reported that 68Ga PSMA PET/CT changed RT management and hormone therapy with an overall change in decision-making in 53.7% of the patients. Dewes et al. [27] analyzed whether the use of 68Ga PSMA PET/CT led to a change of the stage and treatment approach and/or planning. They reported that the actual change in the patient stage and RT concept was 53.3 and 33.3%, respectively. Sterzing et al. [19] also reported 50.8% change in radiotherapeutic management due to the impact of 68Ga PSMA PET/CT in their 57-patient cohort.
The data on the effectiveness of 68Ga PSMA PET/CT-based RT in oligometastatic PC are scarce. Henkenberens et al. [29] evaluated the efficacy of 68Ga PSMA PET/CT for RT in both locally recurrent and oligometastatic PC in 29 patients. In their cohort, 26 patients (89.8%) had high-risk cancer. They reported that 68Ga PSMA PET/CT-guided 3D-conformal RT or IMRT represents a promising treatment option in patients with rising PSA levels in recurrent PC patients and delayed the clinical progression and start of systemic treatment. The authors reported LC rates of 100% for irradiated metastasis for a median follow-up of 8 months. Although a short follow-up period makes it difficult to draw a definite conclusion, an LC rate of 100% was observed in our cohort. In addition, the majority of the patients (73.9%) in our study also presented with initially high-risk disease.
Our study has some limitations. The retrospective nature of the study, the small number of the patients and short follow-up do not allow drawing firm conclusions. Although Henkenberens et al. [29] were the first to investigate the effectiveness of 68Ga PSMA PET-CT-guided RT, we analyzed only oligometastatic PC patients (without local recurrence).
Conclusion
Due to early detection of oligometastatic disease at low PSA values, 68Ga PSMA PET/CT-guided RT may be a promising treatment option in PC patients with biochemical relapse. The current retrospective study showed optimal LC, low toxicity profile and a promising PFS in CS patients with the implementation of moderately fractionated RT. The potential role of RT in oligometastatic PC patients needs to be validated in prospective trials.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355:1491–8.
Ost P, Decaestecker K, Lambert B, Fonteyne V, Delrue L, Lumen N, et al. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate. 2014;74:297–305.
Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:1384–90.
Schweizer MT, Zhou XC, Wang H, Yang T, Shaukat F, Partin AW, et al. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2013;24:2881–6.
Mottet N, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Actas Urol Esp. 2011;35:565–79.
Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol. 2012;2:215.
Berkovic P, De Meerleer G, Delrue L, Lambert B, Fonteyne V, Lumen N, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer. 2013;11:27–32.
Fitzpatrick JM, Bellmunt J, Fizazi K, Heidenreich A, Sternberg CN, Tombal B, et al. Optimal management of metastatic castration-resistant prostate cancer: highlights from a European Expert Consensus Panel. Eur J Cancer. 2014;50:1617–27.
Wright GL Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–34.
Engert A, Raemaekers J. Treatment of early-stage Hodgkin lymphoma. Semin Hematol. 2016;53:165–70.
Onal C, Reyhan M, Guler OC, Yapar AF. Treatment outcomes of patients with cervical cancer with complete metabolic responses after definitive chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1336–42.
Madsen PH, Holdgaard PC, Christensen JB, Hoilund-Carlsen PF. Clinical utility of F-18 FDG PET-CT in the initial evaluation of lung cancer. Eur J Nucl Med Mol Imaging. 2016;43:2084–97.
Mamede M, Ceci F, Castellucci P, Schiavina R, Fuccio C, Nanni C, et al. The role of 11C-choline PET imaging in the early detection of recurrence in surgically treated prostate cancer patients with very low PSA level <0.5 ng/mL. Clin Nucl Med. 2013;38:e342–5.
Evangelista L, Briganti A, Fanti S, Joniau S, Reske S, Schiavina R, et al. New clinical indications for F/C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature. Eur Urol. 2016;70:161–75.
Mease RC, Foss CA, Pomper MG. PET imaging in prostate cancer: focus on prostate-specific membrane antigen. Curr Top Med Chem. 2013;13:951–62.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med Off Publ Soc Nucl Med. 2015;56:668–74.
Sterzing F, Kratochwil C, Fiedler H, Katayama S, Habl G, Kopka K, et al. (68)Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2016;43:34–41.
Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of 68 Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2016;44:92–101.
Deutschmann H, Kametriser G, Steininger P, Scherer P, Scholler H, Gaisberger C, et al. First clinical release of an online, adaptive, aperture-based image-guided radiotherapy strategy in intensity-modulated radiotherapy to correct for inter- and intrafractional rotations of the prostate. Int J Radiat Oncol Biol Phys. 2012;83:1624–32.
Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–9.
Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–94.
Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON, et al. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res. 1999;59:5030–6.
Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–91.
Perner S, Hofer MD, Kim R, Shah RB, Li H, Moller P, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696–701.
Dewes S, Schiller K, Sauter K, Eiber M, Maurer T, Schwaiger M, et al. Integration of (68)Ga-PSMA-PET imaging in planning of primary definitive radiotherapy in prostate cancer: a retrospective study. Radiat Oncol. 2016;11:73.
Shakespeare TP. Effect of prostate-specific membrane antigen positron emission tomography on the decision-making of radiation oncologists. Radiat Oncol. 2015;10:233.
Henkenberens C, von Klot CA, Ross TL, Bengel FM, Wester HJ, Merseburger AS, et al. 68Ga-PSMA ligand PET/CT-based radiotherapy in locally recurrent and recurrent oligometastatic prostate cancer: early efficacy after primary therapy. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al]. 2016;192:431–9.
Acknowledgements
The results of this study were presented in the 22nd National Oncology Congress 19–23 April 2017, Antalya, Turkey, and was awarded the “Best Oral Presentation Award”. Also, it was represented as a poster presentation in ASCO-2017 Genitourinary Cancer Symposium 16–18 February 2017, Orlando, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have stated that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The present work is a retrospective analysis of clinical data.
Rights and permissions
About this article
Cite this article
Guler, O.C., Engels, B., Onal, C. et al. The feasibility of prostate-specific membrane antigen positron emission tomography(PSMA PET/CT)-guided radiotherapy in oligometastatic prostate cancer patients. Clin Transl Oncol 20, 484–490 (2018). https://doi.org/10.1007/s12094-017-1736-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1736-9