Abstract
Purpose
Despite numerous advances, survival remains dismal for children and adolescents with poor prognosis cancers or those who relapse or are refractory to first line treatment. There is, therefore, a major unmet need for new drugs. Recent advances in the knowledge of molecular tumor biology open the door to more adapted therapies according to individual alterations. Promising results in the adult anticancer drug development have not yet been translated into clinical practice. We report the activity in early pediatric oncology trials in Spain.
Methods
All members of the Spanish Society of Pediatric Hematology Oncology (SEHOP) were contacted to obtain information about early trials open in each center.
Results
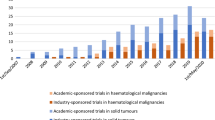
22 phase I and II trials were open as of May 2015: 15 for solid tumors (68 %) and 7 for hematological malignancies (32 %). Fourteen (64 %) were industry sponsored. Since 2010, four centers have joined the Innovative Therapies For Children With Cancer, an international consortium whose aim is developing novel therapies for pediatric cancers. A substantial number of studies have opened in these 5 years, improving the portfolio of trials for children. Results of recently closed trials show the contribution of Spanish investigators, the introduction of molecularly targeted agents and their benefits.
Conclusions
Clinical trials are the way to evaluate new drugs, avoiding the use of off-label drugs that carry significant risks. The Spanish pediatric oncology community through the SEHOP is committed to develop and participate in collaborative academic trials, to favor the advancement and optimization of existing therapies in pediatric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer remains the first cause of death in children. Nonetheless, survival probability has considerably changed within the past 30 years. Clinical research by cooperating groups has progressively increased the long-term survival rate from less than 20 % before 1975 to over 70–80 % in the new millennium [1]. Despite global good results, survival is dismal for children with poor prognosis cancers such as metastatic neuroblastoma, sarcoma, medulloblastoma and high-grade glioma, or those who relapse or are refractory to first line treatment. There is, therefore, an unmet need for new drugs.
New anticancer agents in pediatrics are necessary to improve survival and reduce sequels from current multimodal treatment combining surgery, radiation, chemotherapy and hematopoietic stem cell transplant. More recently, particular molecular alterations have led to the development of personalized targeted therapies, and this opens the horizon for a more adapted therapeutic approach according to individual alterations found at diagnosis or at recurrence.
There are a few pediatric examples where targeted agents have demonstrated their role such as ALK inhibitors in ALK-translocated pediatric anaplastic large cell lymphoma or inflammatory myofibroblastic tumors [2, 3], smoothened inhibition in Hedgehog-driven medulloblastoma [4] or specific BRAF inhibition in BRAFV600E mutated high-grade gliomas [5, 6].
In leukemia, the best two examples are found in chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL), where the finding of the Philadelphia chromosome (t(9;22)(q34;q11)) has allowed the identification of the BCR-ABL fusion protein that plays a central role in the pathogenesis of these diseases. This discovery has permitted the development of novel agents such as imatinib, dasatinib or nilotinib that can eliminate leukemia cells through the inhibition of the ABL kinase activity, significantly improving the outcome of these patients and reducing the need for hematopoietic stem cell transplantation [7, 8].
It is, therefore, of paramount importance to continue investing in research and development of new agents in pediatric oncology.
In 2007, a new regulation for pediatric medicines was implemented in Europe, requiring specific plans for the development of new drugs in children, including anticancer medicines, if the indication is relevant to this population. This new regulation requests the so-called Pediatric Investigational Plan (PIP) that needs to be submitted by the companies to the European Medicines Agency (EMA) at the time of registration of any new drug [9]. This regulation mandates that the pharmaceutical companies propose and comply with a PIP before they seek for marketing authorization (MA) for a new medicine (or variation of an existing MA). Completed PIPs are rewarded with a 6-month extension of the medicine’s supplementary protection certificate or, in the case of orphan designated medicines, a 2-year extension of the 10-year market exclusivity for the authorized indication. The objectives of this new regulation are to increase the availability of authorized medicines for children by generating safety and efficacy data and high-quality ethical pediatric clinical research, and to increase and improve the available information in pediatric drugs [10]. Despite the introduction of these initiatives in the last 15 years in the USA and Europe, new drug development in children with cancer is not sufficient yet: only half of the 28 non-generic cancer drugs approved since 2007 were waived; of these 28 drugs, 26 were potentially relevant for pediatric cancers [11]. It is, therefore, essential that representatives of stakeholders-academia, regulatory bodies, pharmaceutical companies, parents’ organizations and advocacy groups, philanthropic organizations, and government continue to work together and collaborate closely to facilitate the access to new drugs to children with cancer [11].
In general, current pediatric oncology treatments for relapse are often not evidence-based and most of the agents that are used do not have a pediatric indication. Mounting evidence shows that off-label use of adult drugs in pediatrics use has significant risks for the patients and precludes gaining scientific knowledge [12]. The evaluation of new anticancer medicines for children and adolescents should be done within regulatory compliant, ethically approved and well-conducted clinical trials. In this line, the Innovative Therapies For Children With Cancer Consortium (ITCC) (http://www.itcc-consortium.org) is an international organization whose main aim is to evaluate new drugs for the treatment of cancer in children and adolescents. Under this premise, the ITCC provides accreditation for conducting early clinical trials in pediatric cancer and facilitates contact with sponsors to open new sites [13].
The aim of this report is to describe the activity of early (phase I and II) clinical trials for pediatric cancer in Spain. This will serve as a baseline to analyze the early trial activity in the future.
Materials and methods
Phase I and II cancer trials currently open in pediatric centers in Spain were identified through the Spanish Society of Pediatric Hematology and Oncology (SEHOP), an institution that gathers all pediatric oncology units in the country. Last update of this identification process was made in May 2015.
All members of SEHOP were contacted by mail to obtain information about early clinical trials open in each center. This activity is conducted twice a year with the aim of informing all members of all open trials. Additional clinical trial websites such as http://www.clinicaltrials.gov and the EU Clinical Trials Register (http://www.clinicaltrialsregister.eu) were searched.
Results
Early clinical trials centers, role of the ITCC consortium
From 2010, four Spanish centers have progressively joined the Innovative Therapies For Children With Cancer Consortium [13]: Hospital Universitari La Fe in Valencia; Hospital Universitari Vall d’Hebron and Hospital Sant Joan de Déu in Barcelona and Hospital Niño Jesús in Madrid. Other SEHOP centers have also participated in the trials described here, mainly phase II, such as Hospital La Paz and Doce de Octubre in Madrid, Vírgen del Rocío in Seville, Vírgen de la Arrixaca in Murcia, Hospital de Cruces in Bilbao and Hospital General Universitario in Alicante.
Clinical trial portfolio: coverage for most pediatric cancers
Phase I and II trials open in pediatric centers in Spain up to May 2015 are displayed in Tables 1 and 2 for solid tumors and hematological malignancies respectively.
A total of 22 early trials were open. Fifteen early phase clinical trials were open in solid tumors (68 %). Of those, 3 are pure phase I trials (20 %), 5 (33 %) are phase I/II and 7 (47 %) are pure phase II trials. Four studies investigate single or combined cytotoxic agents (27 %) and 1 study a combination of cytotoxic agents ± a monoclonal antibody (MAB); 1 study evaluates oncolytic viral therapy (CELYVIR [14]); 2 trials investigate the combination of two immunotherapeutic agents in neuroblastoma and 1 other a single MAB in melanoma. Six are trials investigating tyrosine kinase inhibitors (TKI) (40 %). Two trials are molecular target specific (13 %), 10 tumor-type specific (67 %) and 3 for all solid tumors (20 %). Six are academic studies (40 %) and 9 are pharmaceutical industry-sponsored (60 %). Ten solid tumor trials are open for patients up to 18 years (67 %), 3 up to 21 years (20 %) and 1 up to 30 years (7 %). One trial only includes patients above 15 years (6 %).
There are 7 early phase clinical trials in hematological malignancies (32 %). Of those, 4 (57 %) are phase I/II and 3 (43 %) are pure phase II trials. Three studies investigate the combination of conventional cytotoxic agents with, either a hypomethylating agent (n = 1; 14 %), a proteasome inhibitor (n = 1; 14 %), a MAB (n = 1; 14 %) or cell therapy with natural killer (NK) cells (n = 1; 14 %). Two are trials investigating a single MAB (29 %) and one a TKI (14 %). One trial (14 %) is the molecular target specific and 6 (86 %) are tumor-type specific. Two are academic studies (29 %) and 5 are pharmaceutical industry sponsored (71 %). Five of these trials are open for patients up to 18 years (71 %) and 2 up to 21 years (29 %).
Table 3 shows the improvement in the options available to treat pediatric patients with relapsed/refractory cancers in Spain from 2010 to 2015.
Trials with results reported
Results of recently closed trials or interim results of ongoing trials have been reported showing the contribution of Spanish investigators, the introduction of molecularly targeted agents into pediatrics and the benefits that individual patients can draw from new agents targeting cancer vulnerabilities (Table 4).
Discussion
This report summarizes the early clinical trial activity in pediatric and adolescent cancers in Spain.
Children with high-risk cancers have difficulties to access novel drugs. More clinical trials have been opened over the past 3 years, probably as a result of joining the ITCC, the pediatric regulation and the efforts of institutions to devote teams dedicated to early clinical trials including physicians, research nurses, study coordinators and data managers.
Between 2010 and 2014, four centers have joined the ITCC, a consortium created in 2003 that gathers 9 European research laboratories and 47 European pediatric oncology units with the capacity to conduct phase I and II cancer trials. One of the objectives of this consortium is to develop novel therapies for the treatment of pediatric and adolescent cancers in collaboration with pharmaceutical companies, regulatory bodies, parents’ and patients’ organizations [13]. In 2013, the ITCC was evaluating twelve new drugs in early phase clinical trials, while it was only one in 2007; half of these trials had been conducted to comply with the regulatory requirements of a PIP; this has notably improved the access of new therapies for children in this continent [10]. As a country, being an active part of the ITCC opens the door to participate in international collaborative studies and facilitate the access of innovative and promising therapies for our patients.
The majority of the trials in pediatric oncology and hematology currently open in our country are sponsored by pharma (64 %). There are fewer trials for indications such as central nervous system (CNS) tumors or sarcomas, a gap that should be bridged through academic-led clinical trials. Through our national Society of Pediatric Hematology and Oncology (SEHOP), we should aim to develop and to participate in collaborative academic trials, to favor the advancement and optimization of already existing therapies in uncommon conditions such as pediatric cancer that often are not a primary focus of for-profit companies. An important caveat at this point is the lack of funding for the conduct of academic clinical trials. There are very few non-profit organizations dedicating specific funding calls for pediatric cancer research and no specific call to support the conduct of clinical trials. For instance, this poses a significant challenge to join international clinical trials where funding for the national and local costs is required.
Besides this, the positive results seen in molecular selected populations treated with targeted agents such as BRAF and ALK inhibitors [3, 4, 6] or anti-GD2 monoclonal antibodies for high-risk neuroblastoma [15] are encouraging. This should stimulate investment in basic and clinical research and to promote addressing patients to centers where innovative therapies are available and that may be of benefit for them.
Recent reports from The Royal Marsden (London, United Kingdom) [16] and Gustave Roussy (Villejuif, France) hospitals [17], two of the largest pediatric oncology phase I/II units in Europe, have shown that implementing early phase clinical trials in pediatric oncology is feasible and safe, and that a significant number of patients will derive benefits in terms of survival.
Clinical trials are the way to evaluate new drugs and gaining robust evidence about treatment efficacy in rare cancers that would benefit doctors and patients alike. Off-label use and compassionate use put patients at increased risk and do not allow gaining scientific knowledge for future patients [12].
This report represents the first effort to describe the activity in pediatric oncology and hematology drug development at a national level. We pretend that this description serves as the baseline to be compared with in the future.
References
Imbach P, Khune T, Arceci J. Introduction: incidence and management of childhood cancer. In: Imbach P, Khune T, Arceci J, editors. Pediatric oncology: a comprehensive guide. Berlin: Springer; 2011. p. XVII–XIX.
Mossé YP, Lim MS, Voss SD, Wilner K, Ruffner K, Laliberte J, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children’s Oncology Group phase 1 consortium study. Lancet Oncol. 2013;14:472–80.
Geoerger B, Schulte J, Zwaan CM, Casanova M, Fischer M, Moreno L, et al. Phase I study of ceritinib in pediatric patients (Pts) with malignancies harboring a genetic alteration in ALK (ALK+): Safety, pharmacokinetic (PK), and efficacy results. J Clin Oncol. 2015;33(suppl):abstr 10005.
Geoerger B, Aerts I, Casanova M, Chisholm J, Hargrave D, Leary S, et al. Phase I/II study of LDE225, a smoothened (Smo) antagonist, in pediatric patients with recurrent medulloblastoma or other solid tumors. J Clin Oncol. 2012;30(suppl):abstr e9519.
Bautista F, Paci A, Minard-Colin V, Dufour C, Grill J, Lacroix L, et al. Vemurafenib in pediatric patients with BRAFV600E mutated high-grade gliomas. Pediatr Blood Cancer. 2014;61:1101–3.
Kieran M, Hargrave D, Cohen Kenneth J, Aerts I, Dunkel I, Ryan Hummel T, et al. Phase 1 study of dabrafenib in pediatric patients (pts) with relapsed or refractory BRAF V600E high- and low-grade gliomas (HGG, LGG), Langerhans cell histiocytosis (LCH), and other solid tumors (OST). J Clin Oncol. 2015;33(suppl):abstr 10004.
Brown P, Hunger SP, Smith FO, Carroll WL, Reaman GH. Novel targeted drug therapies for the treatment of childhood acute leukemia. Expert Rev Hematol. 2009;2:145.
Rives S, Estella J, Gómez P, López-Duarte M, de Miguel PG, Verdeguer A, et al. Intermediate dose of imatinib in combination with chemotherapy followed by allogeneic stem cell transplantation improves early outcome in paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (ALL): results of the Spanish Cooperative G. Br J Haematol. 2011;154:600–11.
Vassal G, Geoerger B, Morland B. Is the European pediatric medicine regulation working for children and adolescents with cancer? Clin Cancer Res. 2013;19:1315–25.
Vassal G, Rousseau R, Blanc P, Moreno L, Bode G, Schwoch S, et al. Creating a unique, multi-stakeholder Paediatric Oncology Platform to improve drug development for children and adolescents with cancer. Eur J Cancer. 2015;51:218–24.
Vassal G, Zwaan CM, Ashley D, Le Deley MC, Hargrave MC, Blanc P, et al. New drugs for children and adolescents with cancer: the need for novel development pathways. Lancet Oncol. 2013;14:e117–24.
Lenk C, Duttge G. Ethical and legal framework and regulation for off-label use: European perspective. Ther Clin Risk Manag. 2014;10:537–46.
Zwaan CM, Kearns P, Caron H, Verschuur A, Riccardi R, Boos J, et al. The role of the “innovative therapies for children with cancer” (ITCC) European consortium. Cancer Treat Rev. 2010;36:328–34.
Ramirez M, Garcıa-Castro J, Alemany R, Melen GJ, Franco L, González-Murillo A, et al. Virotherapy delivered by autologous mesenchymal stem cells for children with metastatic and refractory neuroblastoma: results of a trial of compassionate use. Pediatr Blood Cancer. 2014;61(suppl 2):S107.
Lode HN, Valteau-Couanet D, Garaventa A, Gray J, Castel V, Yaniv I, et al. Long-term infusion of anti-GD2 antibody ch14.18/CHO in combination with interleukin-2 (IL2) activity and efficacy in high-risk relapsed/refractory neuroblastoma patients. J Clin Oncol. 2015;33(suppl):abstr TPS10080.
Morgenstern DA, Hargrave D, Marshall LV, Gatz SA, Barone G, Crowe T, et al. Toxicity and outcome of children and adolescents participating in phase I/II trials of novel anticancer drugs: the Royal Marsden experience. J Pediatr Hematol Oncol. 2014;36:218–23.
Bautista F, Di Giannatale A, Dias-Gastellier N, Fahd M, Valteau-Couanet D, Couanet D, et al. Patients in pediatric phase I and early phase II clinical oncology trials at Gustave Roussy: a 13-year center experience. J Pediatr Hematol Oncol. 2015;37:e102–10.
Acknowledgments
We would like to thank all children and parents who have participated in clinical trials and the clinical staff, research nurses, and research assistants of the different institutions. We are grateful to our colleagues of the Sociedad Española de Hematología y Oncología Pediátrica (SEHOP) for their enthusiasm and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All human studies had been approved by the appropriate ethics committee and had therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Parents and/or legal guardians of children participating in these clinical trials gave their informed consent prior to their inclusion in the studies reported in this manuscript.
Additional information
On behalf of the Sociedad Española de Hematología y Oncología Pediátrica (SEHOP).
Rights and permissions
About this article
Cite this article
Bautista, F., Gallego, S., Cañete, A. et al. Landscape of early clinical trials for childhood and adolescence cancer in Spain. Clin Transl Oncol 18, 708–713 (2016). https://doi.org/10.1007/s12094-015-1421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1421-9