Abstract
Aim
The optimal treatment in older persons with metastatic colorectal cancer (mCRC) is complicated by a lack of general agreement. The aim of this study was to evaluate the activity of bevacizumab plus capecitabine combination in elderly mCRC patients who were not suitable for chemotherapy with irinotecan and oxaliplatin-containing regimens.
Materials and methods
Seventy years and older patients with metastatic colorectal carcinoma were included in this retrospective study. Bevacizumab was administered at a dose of 7.5 mg/kg on day 1 as an intravenous (IV) infusion over 30–90 min every 21 days, and capecitabine was prescribed at 1000 mg/m2 twice daily on days 1–14 of the same 21-day schedule.
Results
Eighty-two consecutive patients (47 men, 35 women) were included in the study. The mean age was 75.5 (SD 3.9, range 70–87). Half of the patients were older than 75 years. There were 55 patients (67.1 %) with a good Eastern Cooperative Oncology Group (ECOG) performance status (PS: 0–1) and the remaining 27 patients (32.9 %) had a poor ECOG performance status (PS: 2). With a median follow-up period of 18.5 months, the median progression-free survival (PFS) was 10 months (95 % CI, 7.8–12.1) and the median OS was 25 months (95 % CI, 18.6–31.3). The main toxicities recorded were non-hematological. Thirty-one patients (37 %) experienced grade 3/4 adverse events, the most common being hand–foot syndrome (9.8 %). No fatal toxicity resulting from this regimen was recorded.
Conclusions
Considering the toxicity profile and survival outcomes, the combination regimen of capecitabine and bevacizumab is a potentially feasible treatment option in elderly mCRC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The survival of patients with metastatic colorectal carcinoma (mCRC) has improved dramatically over the past decade, largely owing to the therapeutic advances including oxaliplatin and irinotecan-containing and molecular targeted therapies [1–4].
The value of therapy is, however, counterbalanced by increased even prohibitive toxicity, particularly among elderly patients who often have co-existing medical conditions and poor performance status. One of the controversies in colon cancer relates to the management of this population. Although advanced age alone is not a consistent predictive value of outcome, elderly mCRC patients may suffer increased risk of side effects due to comorbidities and psychosocial care issues. Many oncologists do preferentially not utilize conventional multiagent chemotherapy in elderly patients, because of concerns about toxicity [5]. However, growing evidence suggests that cancer in older age is often undertreated [6, 7]. Given the limited number of trials there is currently no consensus on the optimal management of elderly mCRC patients and treatment of them remains an area of unmet medical need.
Several trials have been concerned that single agent fluoropyrimidines are better tolerated in the older population [8]. In this ground, the oral prodrug of 5-Fluorouracil, capecitabine offers an attractive alternative to continuous 5-Fluorouracil in terms of relative ease of administration, favorable tolerability and no requirement for premedication [9–11]. Colorectal cancer is among the several malignant tumors that are dependent on angiogenesis to support their growth and metastasis. Bevacizumab is a humanized monoclonal antibody that targets vascular endothelial growth factor (VEGF-A), a major target for anti-angiogenic therapy. It has demonstrated a survival benefit in both the first and second-line treatment of mCRC [2, 12, 13]. Moreover, the survival benefit of adding bevacizumab to fluoropyrimidine-based chemotherapy in patients aged >65 years are comparable with those of the younger population [14].
Treatment options for elderly patients who are not candidates for combination cytotoxic chemotherapy are limited. We performed a retrospective study to investigate the clinical efficacy, tolerability and safety data with the use of capecitabine combined with bevacizumab in the elderly population with mCRC who are not eligible for irinotecan or oxaliplatin-based combination regimens.
Materials and methods
Patients
The study consisted of patients with mCRC who were 70 years or older and given capecitabine plus bevacizumab as first-line treatment due to ineligibility for irinotecan or oxaliplatin-based combination regimens. Medical records of patients were retrospectively reviewed. Clinical and pathologic features including age, gender, performance status (PS), comorbidities, site of primary tumor, K-RAS/N-RAS tumor status, stage at diagnosis, metastatic sites, previous therapies were collected. The PS was graded according to the ECOG scale. Eligible patients were required to have histologically or cytologically proven adenocarcinoma of the colon or rectum. They had to take no prior chemotherapeutic regimen for metastatic disease. Patients initially diagnosed with early or locally advanced stage who subsequently progressed to metastatic disease were also included in the study. Patients relapsed at least 6 months after the completion of adjuvant chemotherapy were allowed. Other inclusion criteria included ineligibility for local therapy, at least one lesion that was measurable by computed tomography or magnetic resonance imaging. Ineligibility criteria included presence of metastasectomy, history of other malignancies except adequately treated basal or squamous cell skin cancer, adjuvant therapy in previous 6 months, uncontrolled hypertension and evidence of clinically significant bleeding diathesis or underlying coagulopathy.
Treatment
Bevacizumab was administered at a dose of 7.5 mg/kg on day 1 as an intravenous (IV) infusion over 30–90 min every 21 days, and capecitabine was prescribed at 1000 mg/m2 twice daily on days 1–14 of the same 21-day schedule. Tumor response was assessed with response evaluation criteria in solid tumors (RECIST) every 9 weeks or sooner if disease progression was suspected. Overall response rate (ORR) was defined as a complete response (CR) or partial response (PR) persisting for at least 4 weeks. Disease control rate was defined as the sum of the ORR and stable disease (SD). Adverse events were assessed according to the National Cancer Institute common terminology criteria for adverse events version 4.0 scale.
Statistical analysis
Overall survival (OS) was calculated as the time from the first day of bevacizumab plus capecitabine treatment until death of any cause. PFS was defined as the interval from the date of the first day of bevacizumab plus capecitabine treatment to the date of objective disease relapse or death from any cause. Data were censored on the date of last contact. Estimates of survival rates were derived by the Kaplan–Meier method and comparisons were made using the log-rank test. SPSS 22.0 statistical software (SPSS Inc, Chicago, IL, USA) was used for all analyses with an alpha level of 5 % considered to indicate statistical significance.
Results
Patient characteristics
Between March 2000 and October 2014, a total of 82 patients (47 men, 35 women) were included in the study. Because not every patient was stage IV at diagnosis, the period of metastatic state started in September 2006. Baseline characteristics of patients are outlined in Table 1. Mean age was 75.5 years (SD ±3.9, range 70–87). Half of the patients were older than 75 years. There were 55 patients (67.1 %) who had good PS (ECOG PS: 0–1). Thirty-seven patients had available K-RAS tumor status. Of these, 14 patients had wild-type K-RAS tumors and 23 had mutant-type K-RAS tumors. At the time of diagnosis, 43 patients (52.4 %) were stage IV. The predominant metastatic site was the liver (61 patients, 74.4 %). Lung metastasis occured in 26 patients (31.7 %). The majority of patients (68.3 %) had at least one comorbidity, the most common of which was arterial hypertension seen in 38 patients (46.3 %).
Treatment
Overall response rate was recorded in 36 patients (43.9 %), including 1 CR (1.2 %) and 35 PR (42.6 %). Twenty-five patients (30.5 %) achieved SD and 21 patients (25.6 %) experienced progressive disease. The rate of tumor control was 74.4 %. The median number of cycles of chemotherapy administered was 6 (3–19). The median relative dose intensity was 92 % and 91.4 % for capecitabine and bevacizumab, respectively. Thirteen patients (15.8 %) went on to receive chemotherapy after disease progression. The additional line of treatment was irinotecan-containing combination chemotherapy in 11 patients and oxaliplatin-containing regimen in 2 patients.
Survival
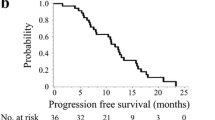
With a median follow-up period of 18.5 months, the median PFS was 10 months (95 % CI 7.8–12.1) and the median OS was 25 months (95 % CI 18.6–31.3). The OS and PFS of patients are provided in Figs. 1 and 2, respectively. Multivariate analysis of clinical factors showed that female gender adversely affects OS (P = 0.039, HR: 0.56 95 % CI 0.333–0.971). However, age, performance status, comorbidities, elevated carcinoembryonic antigen were not significantly prognostic for PFS or OS. Overall survival in relation to gender is presented in Fig. 3.
Toxicity
No patient died as a direct consequence of treatment. The main toxicities recorded were non-hematological. Toxicity was grade 1 or 2 in the majority of cases, with asthenia (50 cases, 60.9 %) representing the most frequent adverse event, followed by hand–foot syndrome (36 cases, 43.9 %) and nausea (30 cases, 36.5 %). Thirty-one patients (37 %) experienced grade 3 or 4 adverse events, the most common being hand–foot syndrome (9.8 %), hypertension (8.5 %), nausea (6.1 %) and diarrhea (6.1 %). Adverse events leading to permanent therapy discontinuation occurred in 9 patients (10.9 %) and reasons were as follows: hypertension (2 cases), gastrointestinal hemorrhage (1 case), cerebrovascular accident (1 case), acute kidney injury (1 case), pulmonary embolism (1 case), diarrhea (1 case), pancreatitis (1 case) and hand–foot syndrome (1 case). For the remaining 22 patients therapy was held and resumed at a 50–75 % dose following resolution of toxicity to grade 2. The occurence of grade 3 hypertension and dose reduction had not been shown to be associated with outcome. Treatment-related toxicities are presented in Table 2.
Discussion
The latest evidence sets a clear mandate for combination therapy with targeted biologic therapies plus 5-Fluorouracil/Leucovorin with either oxaliplatin or irinotecan as the current standard of care for mCRC patients [1–4, 15]. However, the demographic shifts caused by an aging population have led to the emergence of the concept of personalized treatment rather than a ‘one size fits all’ approach. Aging involves a progressive decline in the physiologic capacity of multiple tissues, with increased vulnerability to stress, including cancer treatment. Although the definition of an elderly patient is arbitrary, the incidence of co-existing medical conditions (e.g., comorbid illnesses, frailty, sarcopenia, poor nutrition, geriatric syndromes) that interfere with the treatment of cancer starts to increase after 70 years [16]. Therapeutic complications of major interest involve death, loss of independence, hospitalizations, cognitive impairment, myelosuppression and neutropenic infections.
From the standpoint of metastatic colorectal cancer, increasing attention is being paid to the optimal treatment in older persons by virtue of their increased risk of cancer [17]. For those who are not able to tolerate standard cytotoxic combination chemotherapy as a result of advanced age and/or poor performance status, attempts to enhance the therapeutic ratio have included the use of fluoropyrimidines, with or without targeted agents. For the most part, an oral fluoropyrimidine, capecitabine is the preferred agent because of the convenience of administration and adjustability of doses. The activity of capecitabine in elderly mCRC was evaluated in a phase II trial [18]. Capecitabine produced a PFS of 7 and OS of 11 months. This modest level of efficacy emphasized the need for new treatment options. A first randomized phase II trial [19] of bevacizumab plus capecitabine in elderly mCRC suggested an improvement in PFS and OS with this combination. Their analysis reported a median OS and PFS of 18 months and 10.8 months, respectively. Another phase II trial reported by Vrdoljak et al. [20] indicated that median OS was 21.2 months and PFS was 11.5 months with this combination regimen. Then, the large randomized phase III, AVEX study [21] was undertaken to explore the effect of bevacizumab plus capecitabine combination compared to capecitabine alone in the first-line treatment of elderly mCRC patients. The results showed that survival rates of bevacizumab plus capecitabine were superior to those obtained with capecitabine alone (for OS: 20.7 vs. 16.8 months, for PFS: 9.1 vs. 5.1 months).
The present study, in accordance with previously published data, indicates that bevacizumab combined with capecitabine is associated with improvement in OS and PFS. We found a PFS of 10 months and an OS of 25 months. Our results point to a similar PFS rate with prior studies. The longer OS seen in our trial can be attributed to the confounding effect on OS of post progression therapies. Almost 16 % of patients were able to receive irinotecan or oxaliplatin-containing regimens as subsequent therapy while this ratio was 8 % in the AVEX study [21]. Other possible explanation about favorable outcome in our study is the potential role of prior cytotoxic therapy exposure at adjuvant setting which was 32 % in the AVEX and 14.6 % in our study, as the higher exposure to chemotherapy may cause population of resistant clones that leads to cross-resistance to subsequent similar or dissimilar lines of treatment.
Another concerning finding was our study included 32 % of patients with ECOG PS: 2. Although the mean age and the proportion of patients above 75 years were closely similar, 7 % of patients in the AVEX study [21] and 2 % of patients in the trial of Vrdoljak et al. [20] were with ECOG PS: 2. This indicates that current studies still underestimate the patients with poor performance status. However, some of these patients may derive benefit from therapy as shown in our study.
Among complications of chemotherapy, myelotoxicity is of special concern to elderly, because aging is associated with a more limited reserve of bone marrow stem cells. In the present study, the safety profile of capecitabine was consistent with experience in prior studies; events were predominantly non-haematologic. The most common adverse events were asthenia and hand–foot syndrome. The incidence of grade 3 or 4 adverse events was 37 % in our study which was in the lower range of the former reports. Grade 3 or 4 adverse events were observed in 60 % of patients in the AVEX trial [21]. One potential explanation may relate to the median treatment cycles delivered, which were six in our study whereas were nine in the AVEX trial. A major issue remains arterial hypertension due to bevacizumab use. Seven patients (8.5 %) suffered grade 3 or 4 arterial hypertension, two of whom permanently discontinued treatment in the present study. However, the incidence of grade 3 or 4 arterial hypertension was lower (2 %) in the AVEX trial, reflecting a distinct mechanism that is not related to total treatment cycles delivered.
While some trials has provided evidence in favor of combination cytotoxic chemotherapy in elderly patients [22–24], there are important considerations to be made for the translation of its relevance to the real-world experience. Firstly, most of the studies were restricted to patients with limited comorbidities and stable organ function and cannot be readily extrapolated to all patients with good PS. The 67 % of patients had a PS of 0–1 in our study but while evaluating suitability for combination chemotherapy not only PS was analyzed, instead overall medical fitness, which is a composition of age, PS, comorbidities, frailty, geriatric syndromes and medical history, was taken into account. In the present study, nearly 70 % of patients had at least one comorbidity. Moreover, some of the patients had additional barriers: previous abdominopelvic radiotherapy, hypoalbuminemia, electrolyte imbalance, hyperbilirubinemia, which might be associated with severe irinotecan-related toxicities and sensory neuropathy which precluded oxaliplatin use. Second issue is the patients’ wishes. Patients’ preference of maintaining quality of life is more prominent in this age group compared to younger patients. Thus, the clinicians have a low threshold to proceed with a safer regimen. Targeted therapies offer the efficacy with fewer side effects than cytotoxic chemotherapies. Higher toxicity is, for the most part, a result of cytotoxic chemotherapy. Some studies reported that older patients were subject to increased risk of arterial thromboembolic events due to bevacizumab use compared to younger counterparts [25, 26]. However, in the AGITG MAX trial [27] the addition of bevacizumab to capecitabine in the age over 75-year group was found to be effective with no apparent increase in toxicity compared to younger patients. Similarly, retrospective analysis of pooled cohorts of older patients from two randomized clinical trials [28] found that risks of adding bevacizumab to chemotherapy did not appear to be greater than those seen in younger patients. Nevertheless, greater sensitivity of elderly individuals to thromboembolism due to predisposing medical conditions without having a causal relationship with this treatment cannot be ruled out. Therefore, patients with uncontrolled hypertension, significant bleeding diathesis or underlying coagulopathy were excluded in the present study. Accordingly, a small proportion of patients (2.4 %) suffered from arterial thromboembolism which might also be due to the natural course of the advanced colorectal cancer. Taken together, the addition of bevacizumab without adding a second cytotoxic agent to capecitabine was regarded as safe for elderly patients, provided that special precautions were taken.
Most of the patients whose disease progressed during or after the completion of capecitabine plus bevacizumab received palliative care while 15.8 % were treated with oxaliplatin or irinotecan-containing combination regimens as the next step in the treatment continuum. These regimens were offered to patients who had not aforementioned risk factors for oxaliplatin/irinotecan and who did well on previous line of treatment in terms of toxicity and had a meaningful symptom or PS improvement.
Determinants of prognosis could play a role in optimally individualized treatment concepts. In the multivariate analysis, the female gender was the only factor significantly related to overall survival. Elevated carcinoembryonic antigen, the number of metastases, age and performance status of the patient are the most recognized parameters for the prognostic stratification of metastatic colorectal cancer [29–31]. However, our study failed to demonstrate the significance of these prognostic factors thus could offer the need for further prognostic parameters for the specific subgroup, elderly.
Limitations in this study are mostly related to the retrospective nature of the analysis, which can be subject to selection bias and does not allow for accurate quantification of the severity of the toxicities, particularly the subjective events. Another limitation included missing data for medical comorbid conditions of patients. Finally, the sample size is relatively small.
In conclusion, a considerable proportion of elderly patients with mCRC have reduced performance status or co-existing medical conditions, and these patients are particularly prone to adverse events from combination chemotherapy regimens with oxaliplatin or irinotecan doublets. In our study, which included patients with ECOG PS: 2 (32.9 %) and at least one comorbidity (68 %), the use of capecitabine plus bevacizumab was associated with an improved OS and PFS rates without an excess of severe toxic effects in this challenging population. This finding is in agreement with the already published phase II and phase III studies.
References
Tournigand C, Andre T, Acille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–37.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Van Cutsem E, Nordlinger B, Cervantes A, on behalf of the ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol. 2010;21(suppl 5):v93–7.
Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–8.
Golfinopoulos V, Pentheroudakis G, Pavlidis N. Treatment of colorectal cancer in the elderly: a review of the literature. Cancer Treat Rev. 2006;32:1–8.
Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7.
Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–31.
Scheithauer W, McKendrick J, Begbie S, Borner M, Burns WI, Burris HA, et al. Oral capecitabine as an alternative to i.v. 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol. 2003;14:1735–43.
Schuller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45(4):291–7.
Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19(8):2282–92.
Douillard JY, Sobrero A, Carnaghi C, Comella P, Díaz-Rubio E, Santoro A, et al. Metastatic colorectal cancer: integrating irinotecan into combination and sequential chemotherapy. Ann Oncol. 2003;14(Suppl2):7–12.
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9.
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: results from the Eastern Cooperative Oncology Group (ECOG) study E3200. J Clin Oncol. 2005;23:N16S.
Kozloff MF, Sugrue MM, Purdie DM, Berlin JD, Flynn PJ, Kabbinavar F, et al. Safety and effectiveness of bevacizumab plus chemotherapy in elderly patients with mCRC: results from the BRITE Prospective Cohort Study. J Clin Oncol. 2008;26:184s.
Balducci L. Geriatric oncology: challenges for the new century. Eur J Cancer. 2000;36:1741–54.
National Cancer Institute. Surveillance, epidemiology, and end results. National Cancer Institute: Bethesda; 2007.
Anisimov VN. Biological interactions of aging and carcinogenesis. Cancer Treat Res. 2005;124:17–50.
Feliu J, Escudero P, Llosa F, Bolanos M, Vicent JM, Yubero A, et al. Capecitabine as first-line treatment for patients older than 70 years with metastatic colorectal cancer: an oncopaz cooperative group study. J Clin Oncol. 2005;23(13):3104–11.
Feliu J, Safont MJ, Salud A, Losa F, Garcia-Giron C, Bosch C, et al. Capecitabine and bevacizumab as first-line treatment in elderly patients with metastatic colorectal cancer. Br J Cancer. 2010;102:1468–73.
Vrdoljak E, Omrcen T, Boban M, Hrabar A. Phase II study of bevacizumab in combination with capecitabine as first-line treatment in elderly patients with metastatic colorectal cancer. Anticancer Drugs. 2011;22:191–7.
Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase III trial. Lancet. 2013;14:1077–85.
Sastre J, Marcuello E, Masutti B, Navarro M, Gil S, Antón A, et al. Irinotecan in combination with fluorouracil in a 48-hour continuous infusion as first-line chemotherapy for elderly patients with metastatic colorectal cancer: a Spanish Cooperative Group for the treatment of digestive tumors study. J Clin Oncol. 2005;23(15):3545–51.
Chau I, Norman AR, Cunningham D, Waters JS, Topham C, Middleton G, et al. Elderly patients with fluoropyrimidine and thymidylate synthase inhibitor-resistant advanced colorectal cancer derive similar benefit without excessive toxicity when treated with irinotecan monotherapy. Br J Cancer. 2004;91(8):1453–8.
Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24(25):4085–91.
Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–9.
Kozloff MF, Berlin J, Flynn PJ, Kabbinavar F, Ashby M, Dong W, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRITE observational cohort study. Oncology. 2010;78:329–39.
Price TJ, Zannino D, Wilson K, Simes RJ, Cassidy J, Van Hazel GA, et al. Bevacizumab is equally effective and no more toxic in elderly patients with advanced colorectal cancer: a subgroup analysis from the AGITG MAX trial: an international randomised controlled trial of capecitabine, bevacizumab and mitomycin C. Ann Oncol. 2012;23(6):1531–6.
Kabbinavar FF, Hurwitz HI, Yi J, Sarkar S, Rosen O. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol. 2009;27(2):199–205.
Kronborg CS, Jensen AR. Prognostic factors for overall survival in metastatic colorectal cancer using a stop-and-go FLIRI-based treatment strategy. Int J Colorectal Dis. 2015;30(8):1059–65.
Khanfir A, Feki J, Zidi Z, Masmoudi S, Bayrouti MI, Daoud J, et al. Prognostic factors in metastatic colorectal cancer in Tunisia: a retrospective study of 130 patients. Tunis Med. 2015;93(1):11–5.
Eker B, Ozaslan E, Karaca H, Berk V, Bozkurt O, Inanc M, et al. Factors affecting prognosis in metastatic colorectal cancer patients. Asian Pac J Cancer Prev. 2015;16(7):3015–21.
Acknowledgments
No funders received in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Informed consent
For this retrospective type of study formal consent was not required.
Research involving human participants
No ethics approval was required.
Rights and permissions
About this article
Cite this article
Ozcelik, M., Odabas, H., Ercelep, O. et al. The efficacy and safety of capecitabine plus bevacizumab combination as first-line treatment in elderly metastatic colorectal cancer patients. Clin Transl Oncol 18, 617–624 (2016). https://doi.org/10.1007/s12094-015-1408-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1408-6