Abstract
Backgrounds
Recurrence is the most important factor associated with death of gastric cancer patients after surgery. The aim of this study was to explore the prognosis factors and the effective therapy for recurrent gastric cancer (RGC) patients after radical resection.
Methods
The clinical data of 144 RGC patients who underwent radical resection from January 1999 to March 2004 were reviewed. The 15 clinicopathological factors and treatment modalities on the survival were analyzed. Univariate and multivariate analyses were performed to investigate the prognostic significance of these factors for RGC.
Results
The early recurrence (<2 years) was found in 90 patients, while late recurrence (≥2 years) occurred in 54 patients. The 2-year cumulative survival rates were 23.8 % for recurrent patients receiving chemotherapy plus surgery vs. 1.2 % in patients having chemotherapy only (p < 0.001), while the median survival time was 11.0 months vs. 6.0 months (p < 0.001). Multivariate analysis indicated TNM stage after the first operation (p = 0.048), iASPP overexpression (p = 0.013), time to recurrence (p < 0.001) and treatment of recurrence (p < 0.001) as independent prognostic factors.
Conclusions
Surgery combined with chemotherapy for recurrent gastric cancer patients achieves ideal long-term prognosis, which should perform actively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of gastric cancer has recently been gradually decreasing, and particularly as the advances in diagnostic instruments and more widespread mass screening, the proportion of cases of early gastric cancer increased significantly. However, the prognosis of gastric cancer patients is still dismal, and gastric cancer still remains the fourth most common cancer and the second most frequent cause of cancer death [1–3]. Recurrence is the most important factor associated with death of gastric cancer patients after surgery [4]. Currently, the only radical treatment for gastric cancer is radical gastrectomy. Nevertheless, because more than 50 % of gastric cancer is in the advanced stage at the time of the initial diagnosis, even the curative resection (R0) is possible, the recurrence can occur in about 60 % of patients, through several patterns of dissemination (locoregional, hematogenous, peritoneal) [5–7].
At present, no effective therapy exists for recurrent gastric cancer (RGC). For this reason, numerous studies have investigated the patterns of recurrence [8], the prediction of recurrence [9, 10], and the pre-operative predictive factors of early recurrence [11]. Daniele et al. even try to define a scoring system for the prediction of tumor recurrence after potentially curative surgery for gastric cancer [12]. Unfortunately, the treatment of recurrence remains clinically challenging. Presently, no standard salvage treatment has been established yet for patients in whom recurrence is discovered after radical surgery for gastric cancer [13]. It is very important to predict precisely the factors of prognosis and establish the standard treatment to carry out a better treatment for the RGC patients.
As one member of the apoptosis stimulating proteins of p53 (ASPP) family, iASPP is an evolutionarily conserved inhibitor of p53, and its overexpression correlates with poor prognosis in a variety of tumors [14–16]. However, the relationship between iASPP expression and clinical–pathological characteristics or prognosis of gastric cancer has not been shown.
In the light of these considerations, the aims of the present study were to identify the various recurrence patterns and the prognosis factors, compare and explore the effective therapy for RGC patients following a curative resection.
Patients and methods
Our study protocol was approved by the Institutional Review Board of First Teaching Hospital of Tianjin University of TCM, Tianjin, China.
Study population
The RGC patients who underwent curative gastrectomy between 1999 and 2004 were drawn from the tumor registry. The inclusion criteria for this study included: patients who received a potentially curative resection, and the number of dissected lymph nodes was no <15. The exclusion criteria included: patients who underwent palliative surgery; distant metastasis or peritoneal dissemination was found during the operation; the follow-up data were not completed. Based on these inclusion and exclusion criteria, among total 525 gastric cancer patients, 144 RGC patients were enrolled in this study. 110 patients were male and 34 were female. Their ages ranged from 23 to 85 years with an average age of 56.0 years. The tumor location was: lower third of the stomach in 56 cases (38.9 %), middle third in 16 cases (11.1 %), and upper third in 72 cases (50.0 %). The patients with lymph node metastasis or pT3-4 received adjuvant chemotherapy (intravenous 5-fluorouracil 425 mg/m2 and leucovorin 20 mg/m2 per day for 5 days) and radiotherapy (45 Gy over 25 fractions in 5 weeks). Adjuvant treatment was not given to the 26 (18.1 %) patients.
Data collection
The characteristics, surgical and pathological factors were collected retrospectively from the institute’s gastric cancer database. And the long-term outcome was evaluated by comparing survival rate. The total follow-up time was defined as time in months from the date of operation to last clinic visit or correspondence with the institutional tumor registry. Follow-up was made every 3–6 months for 1–2 years, every 6–12 months for 3–5 years, and a complete history and physical examinations were conducted according to NCCN guide lines.
RNA preparation, quantitative real-time PCR
Total RNA was isolated from frozen tissues using Trizol reagent according to the manufacturer’s instruction. Real-time qRT-PCR was performed according to the manufacturer’s instructions. The primers were as follows:
iASPP: forward 5′-TCTCCTCTGGCCAGCGACCG-3′, reverse 5′-CTGCGAGGCAAAGTGCCCGA-3′; d-glyceraldehyde-3-phosphate dehydrogenase (GAPDH): forward 5′-CCATCAATGACCCCTTCATTG-3′, reverse 5′-GACGGTGCCATGGAATTT-3′. The results of the real-time qRT-PCR were analyzed using the 2−ΔΔCt method. Relative mRNA expression of iASPP gene (R) was calculated using the following formula: R = densitometric units of iASPP/densitometric units of GAPDH. And we found that the median iASPP relative mRNA expression level in tumor specimens was 3.02; we defined high iASPP expression as over 3.02 (>3.02).
Statistical analysis
All continuous data were analyzed by Student’s t test while the categorical variables were analyzed by Chi squared test. The Kaplan–Meier method and log-rank tests were employed to compare survival curves in univariate analysis. Multivariate analyses were conducted using Cox’s proportional hazard regression model. p values (two sides) <0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 15.0 (SPSS, Chicago, IL, USA).
Results
Clinicopathological characteristics
Table 1 summarizes the clinicopathological characteristics of the 144 gastric cancer patients with recurrence following a curative resection. Of these, 22 patients (15.3 %) underwent a total gastrectomy, and the other 122 patients (84.7 %) received a subtotal gastrectomy. 38 patients (26.4 %) were identified as lymph node-negative gastric cancer patients, and the remaining 106 patients (73.6 %) were identified as the lymph node-positive gastric cancer patients. The iASPP mRNA expression was overexpressed in 88 patients. There were no survival differences associated with chemotherapy (p = 0.075). 90 patients (62.5 %) recurred within first 2 years, and 54 patients (37.5 %) recurred ≥2 years after surgery.
iASPP expression was elevated in gastric cancer
The iASPP mRNA expression in gastric cancer tissues was higher than that of control; the median elevated fold was 3.02 (Fig. 2). We also detected the iASPP protein expression of 88 patients, consistent with the mRNA level; the protein expression of iASPP in cancer tissues was also significantly higher than that of non-tumorous tissues (p < 0.05) (Supplement Fig. 1).
Recurrence patterns of gastric cancer patients following a curative resection
The major recurrence patterns are shown in Fig. 1. Overall, 121 patients (84.0 %) had recurrence involving a single pattern, while the other 23 patients (16.0 %) had recurrence involving at least two patterns. The most common pattern was peritoneal recurrence (38.2 %), followed by locoregional recurrence (24.3 %) and hematogenous metastasis (21.5 %). Among the patients, 8 (5.6 %) patients occured locoregional recurrence and distant metastasis concurrently, while 12 (8.3 %) patients developed locoregional recurrence and peritoneal dissemination simultaneously (Fig. 1; Supplementary Table 1).
Among the locoregional recurrence, the most common site was lymph node and followed by proximal resection margin. In hematogenous recurrence, the most common site was the liver, followed by lung, bone, and brain and so on (Supplementary Table 1). Among 63 patients who received the surgery, 51 patients underwent radical surgery, 11 patients received palliative surgical treatment and 1 patient received pure exploration.
Survival outcomes
The survival time of recurrent patients after treatment was from 2 to 37 months, and the 1 and 2 years survival rate was 28.9 and 11.1 %, respectively.
Multivariate analysis was performed on gender, primary tumor size, primary tumor location, gastrectomy, depth of primary tumor invasion, number of metastasized lymph node, TNM stage, MLNR, iASPP expression, time to recurrence, and the treatment of recurrence. It was observed that for the RGC after curative resection, the statistically significant factors were TNM stage after the first operation (p = 0.048), iASPP expression (p = 0.013), time to recurrence (p < 0.001) and treatment of recurrence (p < 0.001) (Table 3).
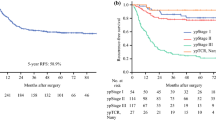
Figure 3 shows cumulative survival curves for the RGC patients receiving chemotherapy plus surgery and patients having chemotherapy only groups. As the figure shows, the 2-year survival rate was significantly better in the surgery plus chemotherapy group than the chemotherapy group (23.8 vs. 1.2 %, respectively; p < 0.001), and the median survival time was 11.0 vs. 6.0 months (p < 0.001).
Discussion
Despite the improved diagnostic methods and extended therapeutic resection, the percent of cases that recur within 2 years after surgery has been reported to be 60–70 % [5–7]. What is more, most cases of mortality are associated with recurrence. Studies on the factors associated with the recurrence of gastric cancer are ongoing, but the information on this is still not sufficient. What is worse, no standard treatment has been established yet for these RGC patients.
The gastric cancer recurrence rate and pattern after curative resection are not uniform as reported [13, 17]. In this current study, 90 patients (62.5 %) recurred within first 2 years (Table 1), and the most common recurrence pattern was peritoneal recurrence, followed by locoregional recurrence and hematogenous metastasis (Fig. 1; Supplementary Table 1). Both the recurrence rate and pattern following curative gastrectomy were similar to other reports [18–20]. However, what are the factors that contribute to recurrence patterns? Recently, Deng et al. retrospectively analyzed the data of 308 gastric cancer patients who underwent a curative resection, and revealed that the Lauren classification was significantly associated with both locoregional recurrence and distant metastasis, while peritoneal dissemination was only associated with N stage.
iASPP has been found to be overexpressed and play an important role in many kinds of human cancers. Consistently, our study showed that the iASPP mRNA expression in gastric cancer tissues was higher than that of control (Fig. 2). In addition, we also detected the iASPP protein expression of 88 patients, consistent with the mRNA level; the protein expression of iASPP in cancer tissues was also significantly higher than that of non-tumorous tissues (p < 0.05) (Supplement Fig. 1). As a new identified oncoprotein, iASPP can promote cell proliferation, inhibit cell apoptosis [21, 22]. Recently, the potential prognostic value of iASPP has been identified in ovarian cancer, hepatocellular carcinoma, head and neck squamous cell carcinoma, and the early stage cervical cancer [16, 23–25]. In the present study, our data showed that the 1- and 2-year survival rate was significantly shorter in the high iASPP expression group compared to low expression group (Table 2). Further multivariate Cox analysis confirmed that high iASPP expression was an independent poor prognostic factor for long-term outcome in patients with RGC (Table 3).
There are no specific treatments to avoid recurrence of gastric cancer. A curative resection, a standard lymphadenectomy and adjuvant therapy following a curative resection are important strategies to prevent recurrence after surgery. However, these points still remain controversial [26–30]. Therefore, the prevention of recurrence as well as the early detection of recurrence is important. Among various clinicopathological factors, efforts should be made to find new predictive factors that are clearly correlated with the recurrence of gastric cancer. In the present study, TNM stage after the first operation (p = 0.048), time to recurrence (p < 0.001) and treatment of recurrence (p < 0.001) were observed to be independent prognostic factors in predicting the prognosis of RGC (Table 3). As the Table 2 shows, patients with more advanced stage of disease have worse prognosis, which confirmed the Shiraishi’s result [10]. Also patients with early recurrence have poorer survival compared with those with later recurrence after curative gastrectomy. Median survival times after recurrence were 5.0 and 12.0 months in the early (recurrence within 2 years) and late (recurrence after >2 years) groups, respectively (Table 2). In addition to these determined clinicopathological factors, efforts have recently been made to apply molecular makers to find predictive factors for recurrence after radical resection of gastric cancer. Kim et al. reported that the expression of c-erb B2 was significantly higher in the recurrence group (p = 0.024) [31].
Based on these results, if appropriate follow-up observation is performed, recurrence may be detected early and then properly treated, and the mortality rate of gastric cancer itself could be decreased. Unfortunately, at present, no effective therapy exists for RGC. Although there is no consensus on the proper indications for surgery in patients with recurrence, long-term survival can be expected when complete resection is accomplished after multidisciplinary assessment. An approximately 20 % 5-year survival can be expected with complete resection [32]. The median survival time after surgery for recurrent patients was reported generally longer than that for recurrent patients who had been treated with systemic chemotherapy alone [33–35]. In our study, 63 recurrent patients receive chemotherapy plus surgery, while 81 patients have chemotherapy only (Table 2). As the Fig. 3 shows, the 2-year survival rate was significantly better in the surgery plus chemotherapy group than in the chemotherapy group (23.8 vs. 1.2 %, respectively; p < 0.001). Therefore, for resectable lesions, aggressive surgical approaches are strongly recommended.
The cumulative survival curves for the recurrent patients receiving chemotherapy plus surgery and patients having chemotherapy only groups. As the figure shows, the 2-year survival rate was significantly better in the surgery plus chemotherapy group than in the chemotherapy group (23.8 vs. 1.2 %, respectively; p < 0.001)
This study has several potential limitations. Firstly, for the iASPP protein expression, we only have 88 patients’ data. And as we have relative small-size patients in this study, further large-size study needs to be conducted to validate our result.
Conclusions
In conclusion, the findings of this study suggest that TNM stage after the first operation, iASPP expression, time to recurrence and treatment of recurrence were independent prognostic factors for RGC. Surgery combined with chemotherapy should perform actively for RGC patients.
References
Matsukuma A, Furusawa M, Tomoda H, Seo Y. A clinicopathological study of asymptomatic gastric cancer. Br J Cancer. 1996;74:1647–50.
Liu C, Zhang R, Lu Y, Li H, Lu P, Yao F, et al. Prognostic role of lymphatic vessel invasion in early gastric cancer: a retrospective study of 188 cases. Surg Oncol. 2010;19:4–10.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Xu AM, Huang L, Zhu L, Wei ZJ. Significance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 cases. Am J Cancer Res. 2014;4:189–95.
Buzzoni R, Bajetta E, Di Bartolomeo M, Miceli R, Beretta E, Ferrario E, et al. Pathological features as predictors of recurrence after radical resection of gastric cancer. Br J Surg. 2006;93:205–9.
Cidon EU. Gastric cancer and the search for a good prognostic classification: a challenge. Clin Exp Gastroenterol. 2010;3:113–6.
Xu DZ, Geng QR, Long ZJ, Zhan YQ, Li W, Zhou ZW, et al. Positive lymph node ratio is an independent prognostic factor in gastric cancer after d2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol. 2009;16:319–26.
Bilici A, Selcukbiricik F. Prognostic significance of the recurrence pattern and risk factors for recurrence in patients with proximal gastric cancer who underwent curative gastrectomy. Tumour Biol. 2015. doi:10.1007/s13277-015-3304-7.
Choi JY, Ha TK, Kwon SJ. Clinicopathologic characteristics of gastric cancer patients according to the timing of the recurrence after curative surgery. J Gastric Cancer. 2011;11:46–54.
Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 2000;89:255–61.
Mal F, Perniceni T, Levard H, Denet C, Validire P, Gayet B. Pre-operative predictive factors of early recurrence after resection of adenocarcinoma of the esophagus and cardia. Gastroenterol Clin Biol. 2005;29:1275–8.
Marrelli D, De Stefano A, de Manzoni G, Morgagni P, Di Leo A, Roviello F. Prediction of recurrence after radical surgery for gastric cancer: a scoring system obtained from a prospective multicenter study. Ann Surg. 2005;241:247–55.
Li JH, Zhang SW, Liu J, Shao MZ, Chen L. Review of clinical investigation on recurrence of gastric cancer following curative resection. Chin Med J (Engl). 2012;125:1479–95.
Lu B, Guo H, Zhao J, Wang C, Wu G, Pang M, et al. Increased expression of iASPP, regulated by hepatitis B virus X protein-mediated NF-kappaB activation, in hepatocellular carcinoma. Gastroenterology. 2010;139(2183–2194):e2185.
Chen J, Xie F, Zhang L, Jiang WG. iASPP is over-expressed in human non-small cell lung cancer and regulates the proliferation of lung cancer cells through a p53 associated pathway. BMC Cancer. 2010;10:694.
Kong F, Shi X, Li H, Li P, Yu J, Li X, et al. Increased expression of iASPP correlates with poor prognosis in FIGO IA2-IIA cervical adenocarcinoma following a curative resection. Am J Cancer Res. 2015;5:1217–24.
La Torre M, Rossi Del Monte S, Ferri M, Cosenza G, Mercantini P, Ziparo V. Peritoneal washing cytology in gastric cancer. How, when and who will get a benefit? A review. Minerva Gastroenterol Dietol. 2011;57:43–51.
Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353–7.
Wu CW, Lo SS, Shen KH, Hsieh MC, Chen JH, Chiang JH, et al. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg. 2003;27:153–8.
Gunderson LL. Gastric cancer–patterns of relapse after surgical resection. Semin Radiat Oncol. 2002;12:150–61.
Li G, Wang R, Gao J, Deng K, Wei J, Wei Y. RNA interference-mediated silencing of iASPP induces cell proliferation inhibition and G0/G1 cell cycle arrest in U251 human glioblastoma cells. Mol Cell Biochem. 2011;350:193–200.
Liu T, Li L, Yang W, Jia H, Xu M, Bi J, et al. iASPP is important for bladder cancer cell proliferation. Oncol Res. 2011;19:125–30.
Jiang L, Siu MK, Wong OG, Tam KF, Lu X, Lam EW, et al. iASPP and chemoresistance in ovarian cancers: effects on paclitaxel-mediated mitotic catastrophe. Clin Cancer Res. 2011;17:6924–33.
Liu Z, Zhang X, Huang D, Liu Y, Liu L, Li G, et al. Elevated expression of iASPP in head and neck squamous cell carcinoma and its clinical significance. Med Oncol. 2012;29:3381–8.
Cao L, Huang Q, He J, Lu J, Xiong Y. Elevated expression of iASPP correlates with poor prognosis and chemoresistance/radioresistance in FIGO Ib1-IIa squamous cell cervical cancer. Cell Tissue Res. 2013;352:361–9.
Irvin TT, Bridger JE. Gastric cancer: an audit of 122 consecutive cases and the results of R1 gastrectomy. Br J Surg. 1988;75:106–9.
Heberer G, Teichmann RK, Kramling HJ, Gunther B. Results of gastric resection for carcinoma of the stomach: the European experience. World J Surg. 1988;12:374–81.
Dent DM, Madden MV, Price SK. Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg. 1988;75:110–2.
Miwa K, Miyazaki I, Sahara H, Fujimura T, Yonemura Y, Noguchi M, et al. Rationale for extensive lymphadenectomy in early gastric carcinoma. Br J Cancer. 1995;72:1518–24.
Plukker JT, Kampschoer GH. Extended lymph-node dissection for gastric cancer: a challenge for better survival results. Neth J Surg. 1990;42:3–8.
Kim JW, Hwang I, Kim MJ, Jang SJ. Clinicopathological characteristics and predictive markers of early gastric cancer with recurrence. J Korean Med Sci. 2009;24:1158–64.
Lehnert T, Rudek B, Buhl K, Golling M. Surgical therapy for loco-regional recurrence and distant metastasis of gastric cancer. Eur J Surg Oncol. 2002;28:455–61.
de Liano AD, Yarnoz C, Aguilar R, Artieda C, Ortiz H. Surgical treatment of recurrent gastric cancer. Gastric Cancer. 2008;11:10–4.
Song KY, Park SM, Kim SN, Park CH. The role of surgery in the treatment of recurrent gastric cancer. Am J Surg. 2008;196:19–22.
Tanizawa Y, Bando E, Kawamura T, Tokunaga M, Kondo J, Taki Y, et al. Influence of a positive proximal margin on oral intake in patients with palliative gastrectomy for far advanced gastric cancer. World J Surg. 2011;35:1030–4.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81273937 and No. 81403220).
Conflict of interest
All authors have no potential conflicts of interest.
Ethical approval
Our study protocol was approved by the Institutional Review Board of First Teaching Hospital of Tianjin University of TCM, Tianjin, China.
Informed consent
Written informed consent was obtained from all patients who participate in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Kong and Y. Qi contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12094_2015_1327_MOESM1_ESM.tif
Supplement Fig. 1 The iASPP protein expression in gastric cancer. The protein expression of iASPP in cancer tissues was significantly higher than that of non-tumorous tissues (p < 0.05) (TIFF 57 kb)
Rights and permissions
About this article
Cite this article
Kong, F., Qi, Y., Liu, H. et al. Surgery combined with chemotherapy for recurrent gastric cancer achieves better long-term prognosis. Clin Transl Oncol 17, 917–924 (2015). https://doi.org/10.1007/s12094-015-1327-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1327-6