Abstract
Background
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related deaths worldwide. Gastric cancer is characterized by high levels of invasion and metastasis. Increasing attention is being focused on discovering molecular markers for the diagnosis of gastric cancer and for predicting its prognosis. The objective of the present study was to evaluate Nurr1 expression in gastric cancer and to assess its correlation with clinicopathological parameters and prognosis in gastric cancer patients.
Methods
Tissue samples were obtained from 120 gastric cancer patients. We investigated Nurr1 expression in human normal and gastric cancer tissues using real-time reverse transcription polymerase chain reaction (qRT-PCR), western blotting, and immunohistochemistry. We determined the association between Nurr1 and recurrence, prognosis and patient clinicopathological parameters. Univariate and multivariate survival analyses with a Cox’s proportional hazards regression model were used to identify independent factors related to recurrence and prognosis.
Results
The immunohistochemical, qRT-PCR and western blot analyses revealed that Nurr1 expression was increased in gastric cancer tissues compared with normal gastric tissue (P < 0.05). Nurr1 expression was significantly correlated with the tumor size, depth of tumor invasion, lymph node metastasis, recurrence, and distant metastasis of gastric cancer (P < 0.05). Moreover, Nurr1-high patients also exhibited poorer overall survival (OS) and disease-free survival compared with Nurr1-low patients (P < 0.01). The univariate and multivariate survival analyses suggested that Nurr1 expression (P = 0.011), histology (P = 0.018), depth of tumor invasion (P = 0.037), and presence of lymph node metastasis (P = 0.031) were independent prognostic factors for recurrence. In addition, Nurr1 expression (P = 0.007), depth of tumor invasion (P = 0.014), lymph node metastasis (P = 0.044), distant metastasis (P = 0.023), and recurrence (P = 0.011) were independent prognostic factors of OS in gastric cancer patients.
Conclusions
The Nurr1 protein may be useful as a marker of recurrence, metastasis, and poor prognosis following curative resection in patients with gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth most common cancer and the second leading cause of cancer deaths worldwide despite the fact that its incidence and mortality have been appreciably declining for several decades. East Asian countries, including China, are among the highest risk areas for gastric cancer [1–3]. Gastric cancer is one of the three most common cancers in China. Approximately 300,000 deaths in China occur annually [4, 5]. Most of the patients are in advanced stages when diagnosed. Given the high incidence of recurrence and metastasis, overall survival (OS) remains poor even after surgical resection and/or systemic chemotherapy, and some patients exhibit an unfavorable course despite receiving curative resection [6]. A favorable prognosis is expected for gastric cancer patients following curative surgery for early-stage disease. Therefore, detecting gastric cancer early and preventing cancer from disseminating is very important. In addition to various critical clinicopathological parameters, the prognosis of gastric cancer is influenced by several biological variables. Predicting patient prognosis and providing promising therapeutic targets is also crucial for decreasing mortality. Thus, in this context, it is necessary to identify novel cancer-related factors that can be used as markers for the diagnosis and treatment of gastric cancer.
Nurr1 (NOT/NR4A2) belongs to the NR4A family of nuclear receptors and is important in a number of biological processes, including regulation of proliferation, apoptosis, migration, and differentiation [7]. Recently, the oncogenic activities of Nurr1 have been uncovered. Nurr1 may suppress apoptosis in breast cancer cells [8] and is an independent prognostic marker for tumor progression and survival in patients with bladder, prostate, and breast cancer [8–10]. Increased levels of prostaglandin E2 (PGE2) are significantly associated with the progression of colorectal cancer. PGE2 can induce Nurr1 expression in colorectal cancer, further supporting its role in tumor progression [11].
To investigate whether Nurr1 is involved in the development and progression of gastric cancer, we evaluated its expression by western blotting and real-time reverse transcription polymerase chain reaction (qRT-PCR) analyses using normal human gastric mucosa and adenocarcinoma samples. We also evaluated Nurr1 expression in gastric cancer samples by immunohistochemistry. Finally, we assessed the association between Nurr1 expression and the clinicopathological parameters and survival of gastric cancer patients.
Materials and methods
Patient specimens
This retrospective study enrolled a consecutive series of 120 gastric cancer patients. All patients were diagnosed and treated at the First Affiliated Hospital of Dalian Medical University (Dalian, China) between September 2004 and September 2014. The exclusion criteria included previous chemotherapy or radiotherapy; hepatic, renal, pulmonary, or cardiac dysfunction; severe complications, such as an anastomotic fistula, during the perioperative period, which was defined as from 7 days before operation until postoperative day (POD) 28; less than 15 lymph nodes retrieved; and loss to follow-up.
In total, 72 men and 48 women enrolled in this study, and patient age ranged from 24 to 86 years old (mean age, 65.38 ± 13.71 years old). Among the 120 patients, 37, 11, 69, and three patients received distal, proximal, total, and partial gastrectomy, respectively. The numbers of patients with stage I, II, and III gastric cancers were 24, 32, and 64, respectively. Histopathologically, 53 cases were differentiated (papillary, well-differentiated, and moderately differentiated tubular adenocarcinoma) and 67 were undifferentiated (poorly differentiated adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma) carcinomas according to the 7th edition of the tumor node metastasis (TNM) classification. The main clinicopathological parameters of all patients are presented in Table 1. The mean follow-up time was 52.33 months (range, 4–120 months). By the end of this study, 52 of the 120 patients died. The patients received follow-up by direct evaluation or phone interviews until death or September 2014.
All tissue samples were fixed in formalin and embedded in paraffin. The clinicopathological parameters examined included age, gender, tumor size, histological grade, depth of invasion, lymph node metastasis, distant metastasis, American Joint Committee on Cancer (AJCC) stage, and patient survival.
RNA extraction and qRT-PCR
Total RNA was extracted using the TRIzol reagent (TaKaRa, Dalian, China). cDNA was synthesized with the TransScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR Kit (TransGen Biotech, Beijing, China). Nurr1 mRNA levels were determined by a reverse transcription PCR analysis using the following primers: Nurr1, 5′-CCTGACTTCAGATCAAGAGACGGTA-3′ and 5′-CTGATTAAAAATGTCTCCACGAACAG-3′; β-actin, 5′-GTGGGGCCCCAGGCACCA-3′ and 5′-CTTCCTTAATGTCACGCACGATTTC-3′. Nurr1 and β-actin primers were provided by Invitrogen (Shanghai, China). Real-time PCR analyses were performed to detect mRNA expression using a TransStart Top Green qPCR SuperMix kit (TransGen Biotech, Beijing, China). Samples were run in a 7500 Fast Real-time PCR System (Applied Biosystems), and β-actin was used as an internal control.
Western blot analysis
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12 % gels and then transferred to nitrocellulose membranes (Millipore). After blocking with 5 % non-fat milk, the membranes were incubated with rabbit anti-Nurr1 monoclonal antibodies (1:500, Santa Cruz, USA) and mouse anti-β-actin antibodies (1:1000, TransGen Biotech, Beijing, China). The proteins were visualized using ECL reagents (Beyotime, Jiangsu, China).
Immunohistochemistry of Nurr1 in gastric cancer tissue
Samples were fixed with 10 % formaldehyde, embedded in paraffin, sectioned into 4-μm-thick sections, and mounted on glass slides. The sections were deparaffinized in xylene and dehydrated in a series of graded ethanol. For staining with anti-Nurr1 antibodies, the sections were pretreated with citrate buffer for 10 min at 121 °C in a microwave oven to retrieve antigens. The sections were then incubated in 3 % hydrogen peroxide for 10 min at room temperature and treated with 1 % goat serum albumin for 30 min at room temperature to block nonspecific reactions. The blocked sections were incubated with the diluted primary anti-Nurr1 antibody (1:200, Santa Cruz, USA) in PBS at 4 °C overnight followed by staining with a streptavidin–biotin–peroxidase kit (ZSGB-bio, Beijing, China). The immune complex was visualized by incubating the sections with diaminobenzidine tetrahydrochloride. Samples were counterstained with hematoxylin and mounted using a permanent mounting medium. Nurr1 expression was determined by counting the number of cells that were positively stained. All slides stained with Nurr1 were reviewed independently by two of the authors with no knowledge regarding the clinical data. The staining intensity of Nurr1 was scored as 0 (none), 1+ (weak), 2+ (moderate), or 3+ (strong) according to previous studies [8, 10]. When heterogeneity was observed, the evaluation was performed in the predominant area. Scores of 0 and 1+ were defined as Nurr1-low, and scores of 2+ and 3+ were defined as Nurr1-high.
Statistical analysis
The statistical analysis was performed using the SPSS software program (version 15.0; SPSS Inc., Chicago, IL, USA). The χ 2 and Mann–Whitney U tests were used to examine the associations between Nurr1 expression and various clinicopathological parameters. Kaplan–Meier analysis with a log-rank test was used to calculate the OS curves. Univariate and multivariate survival analyses with Cox’s proportional hazard regression model were used to evaluate the independent prognostic factors. P values <0.05 were considered significant.
Results
Nurr1 expression is frequently increased in human gastric cancer tissues
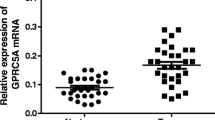
First, we analyzed Nurr1 expression in twenty-four paired gastric cancer and adjacent non-tumor tissues by a qRT-PCR analysis and western blotting. Nurr1 was significantly upregulated in gastric cancer tissues (Fig. 1a, b). Additionally, Nurr1 expression was evaluated in normal and gastric cancer tissues by immunohistochemistry (Fig. 2). We observed increased Nurr1 expression in approximately all gastric cancer samples compared with normal gastric tissues. Therefore, our results demonstrated that Nurr1 expression is upregulated in gastric cancer tissues.
Nurr1 expression in gastric carcinoma tissues and normal tissues. Nurr1 protein expression was assayed by a qRT-PCR and b western blotting. Nurr1 mRNA and protein expression levels in gastric cancer tissues were increased compared with normal tissues. *P < 0.01 compared with the control group. NOR normal gastric tissue group, GC gastric cancer tissue group
Nurr1 immunohistochemical staining in clinical gastric cancer tissues, tissues adjacent to gastric cancers, and normal gastric tissue samples. Representative immunohistochemical staining of Nurr1 in a normal gastric tissue; b tissue adjacent to gastric cancer tissue; c gastric cancer tissue (original magnification, ×400; Scale bar 50 µm)
Nurr1 expression is correlated with metastasis and recurrence of gastric cancer
A strong correlation between Nurr1 expression and the depth of tumor invasion was noted. Furthermore, Nurr1 expression was more frequently increased in advanced-stage tumors and tumors with lymph node or distant metastases compared with early-stage tumors and those without metastasis. No significant differences were noted in Nurr1 expression based on age, gender, histological grade, or tumor size (Table 1).
Nurr1 overexpression is associated with tumor recurrence
A univariate analysis was performed to identify the factors significantly associated with tumor recurrence. As shown in Table 2, lymph node involvement, TNM stage, and extent of tumor differentiation were associated with recurrence. In addition, a high Nurr1 level was associated with a high recurrence rate. The multivariate analysis indicated that lymph node involvement, TNM stage, tumor differentiation, and Nurr1 expression were independent prognostic factors for tumor recurrence (Table 2).
Nurr1 overexpression is associated with a poor OS and DFS
Disease-free survival (DFS) was defined as the length of time from curative surgery to the first tumor recurrence or distant metastasis. OS was defined as the time from curative surgery to death from any cause. We next examined the effects of Nurr1 expression on patient survival. As expected, Nurr1 overexpression was significantly correlated with a poor OS (P = 0.009, log-rank test) (Fig. 3a) and a poor DFS (P = 0.002, log-rank test) (Fig. 3b). Moreover, Kaplan–Meier survival curves exhibited a significant difference in OS and DFS based on Nurr1 expression.
Postoperative outcomes of the 120 gastric cancer patients based on Nurr1 expression. The OS and DFS rates of 120 patients with gastric cancer were analyzed by Kaplan–Meier analysis based on Nurr1 expression. Survival curves were constructed for the 120 patients who were divided into Nurr1-high and Nurr1-low groups. The Nurr1-high group exhibited a significantly poorer outcome compared with the Nurr1-low group (P = 0.009)
Table 3 depicts the results of the univariate and multivariate analyses of factors related to prognosis in patients with gastric cancer. Univariate analysis demonstrated that the depth of tumor invasion, lymph node metastasis, distant metastasis, recurrence, and Nurr1 expression were significantly related to OS (all P < 0.05). The multivariate analysis also suggested that Nurr1 expression (P = 0.007), depth of tumor invasion (P = 0.014), lymph node metastasis (P = 0.044), distant metastasis (P = 0.023), and recurrence (P = 0.011) were independent prognostic factors for the OS of gastric cancer patients.
Discussion
The majority of gastric cancer deaths result from tumor recurrence and metastases rather than primary tumors [12]. However, the molecular mechanisms regulating the invasion and metastasis of gastric cancer remain incompletely understood. Previous studies demonstrated that Nurr1 exhibits oncogenic activities in a cell type-specific manner [10, 13]. Our systematic study of the Nurr1 protein in normal and malignant gastric tissues revealed that Nurr1 was expressed in both normal and gastric cancer tissues. Nurr1 expression in many of the benign tissue specimens was low, whereas high levels of expression were observed in the gastric cancer tissues. Kaplan–Meier analyses revealed that patients with high Nurr1 expression exhibited poorer prognoses and a shorter OS compared with those with low Nurr1 expression. Nurr1 overexpression was frequently detected in our gastric cancer tissue specimens, and the frequency of Nurr1 overexpression increased as TNM classification and the recurrence of gastric cancer increased. These findings suggest the possibility that increased Nurr1 expression may provide a selective advantage supporting the occurrence and progression of gastric cancer.
Increased Nurr1 expression in tumors is associated with unsatisfactory therapeutic outcomes in cancer patients in previous studies [14]. Supporting the role of Nurr1 in cancer progression, Nurr1 knockdown inhibited the proliferation of PC-3 prostate cancer cells [10]. Nurr1-dependent transcription of cell motility-related proteins is a plausible mechanism underlying the Nurr1-induced cell proliferation, invasion, and migration, but the exact mechanism remains uncharacterized [15]. These data support the hypothesis that Nurr1 overexpression correlated with a poor prognosis and reduced survival in gastric cancer patients. However, it is likely that the regulatory mechanisms affecting Nurr1 are intricate, complex, and highly dynamic, varying according to the different genetic and epigenetic contexts of cells and tissues. In addition, the retrospective design of the present study, with its inherent deficiency in the variability of data recording, is an important limitation of the study. Another limitation of this investigation involves its small sample size. A larger scale, multicenter review would allow our conclusions to be confirmed. These findings warrant further investigations into the specific functions of Nurr1 in various types of cancers.
In the present study, we found that Nurr1 was mainly present in cancer epithelial cells and that its expression varied considerably between normal gastric epithelial tissues and gastric cancer tissues. Our investigation revealed pronounced Nurr1 expression in both normal gastric and cancer tissues, but Nurr1 overexpression was correlated with a larger tumor size, greater depth of tumor invasion, lymph node metastasis, and/or recurrence. These data suggest that Nurr1 may be involved in tumor progression. However, we were unable to identify any correlation of Nurr1 expression with age or gender (Table 1).
Nurr1 was recently reported to have anti-apoptotic effects [16]. Nurr1 reduces the expression of the pro-apoptotic protein Bax and interacts with p53 to suppress its transcriptional activity [17]. Overexpression of Nurr1 also leads to downregulation of caspase-3 and other apoptotic factors in neural cells [18]. Knockdown of endogenous Nurr1 expression also attenuates the vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation, migration, and in vivo angiogenesis in Matrigel, suggesting its potential importance in mediating VEGF-induced tumor angiogenesis [19]. PGE2 inhibits apoptosis by inducing Nurr1 expression in colorectal cancer [11]. These studies as well as our present findings support an oncogenic role for Nurr1, which appears to promote cancer development and progression.
In conclusion, our study demonstrated that Nurr1 may be a useful molecular marker and an indicator for gastric cancer progression. In combination with other biomarkers of gastric cancer, Nurr1 would be useful for determining the prognosis of the disease and may also represent a novel therapeutic target. Further studies are needed to investigate the mechanisms and pathways of gastric cancer pathogenesis mediated by Nurr1.
References
Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125(3):666–73.
Bosetti C, Bertuccio P, Malvezzi M, Levi F, Chatenoud L, Negri E, et al. Cancer mortality in Europe, 2005–2009, and an overview of trends since 1980. Ann Oncol. 2013;24(10):2657–71.
Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, et al. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50(7):1330–44.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer incidence and mortality worldwide: IARC Cancer Base No. 10. http://globocan.iarc.fr, 2010. Accessed 15 June 2013.
Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14(12):e535–47.
Sekiguchi M, Suzuki H, Oda I, Abe S, Nonaka S, Yoshinaga S, et al. Risk of recurrent gastric cancer after endoscopic resection with a positive lateral margin. Endoscopy. 2014;46(4):273–8.
Wallén A, Zetterström RH, Solomin L, Arvidsson M, Olson L, Perlmann T. Fate of mesencephalic AHD2-expressing dopamine progenitor cells in NURR1 mutant mice. Exp Cell Res. 1999;253(2):737–46.
Llopis S, Singleton B, Duplessis T, Carrier L, Rowan B, Williams C. Dichotomous roles for the orphan nuclear receptor NURR1 in breast cancer. BMC Cancer. 2013;13:139.
Inamoto T, Czerniak BA, Dinney CP, Kamat AM. Cytoplasmic mislocalization of the orphan nuclear receptor Nurr1 is a prognostic factor in bladder cancer. Cancer. 2010;116(2):340–6.
Wang J, Yang J, Zou Y, Huang GL, He ZW. Orphan nuclear receptor nurr1 as a potential novel marker for progression in human prostate cancer. Asian Pac J Cancer Prev. 2013;14(3):2023–8.
Holla Vijaykumar R, Mann Jason R. Qiong. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J Biol Chem. 2006;281(5):2676–82.
Nomura E, Lee SW, Bouras G, Tokuhara T, Hayashi M, Hiramatsu M, et al. Functional outcomes according to the size of the gastric remnant and type of reconstruction following laparoscopic distal gastrectomy for gastric cancer. Gastric Cancer. 2011;14(3):279–84.
Deutsch AJ, Angerer H, Fuchs TE, Neumeister P. The nuclear orphan receptors NR4A as therapeutic target in cancer therapy. Anticancer Agents Med Chem. 2012;12(9):1001–14.
Wang J, Yang J, Li BB, He ZW. High cytoplasmic expression of the orphan nuclear receptor NR4A2 predicts poor survival in nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2013;14(5):2805–9.
Inamoto T, Czerniak BA, Dinney CP, Kamat AM. Cytoplasmic mislocalization of the orphan nuclear receptor Nurr1 is a prognostic factor in bladder cancer. Cancer. 2010;116(2):340–6.
Boakye CH, Doddapaneni R, Shah PP, Patel AR, Godugu C, Safe S, et al. Chemoprevention of skin cancer with 1,1-Bis (3′-indolyl)-1-(aromatic) methane analog through induction of the orphan nuclear receptor, NR4A2 (Nurr1). PLoS ONE. 2013;8(8):e69519.
Zhang T, Wang P, Ren H, Fan J, Wang G. NGFI-B nuclear orphan receptor Nurr1 interacts with p53 and suppresses its transcriptional activity. Mol Cancer Res. 2009;7(8):1408–15.
Imam SZ, Jankovic J, Ali SF, Skinner JT, Xie W, Conneely OM, et al. Nitric oxide mediates increased susceptibility to dopaminergic damage in Nurr1 heterozygous mice. FASEB J. 2005;19(11):1441–50.
Zhao D, Desai S, Zeng H. VEGF stimulates PKD-mediated CREB-dependent orphan nuclear receptor Nurr1 expression: role in VEGF-induced angiogenesis. Int J Cancer. 2011;128(11):2602–12.
Conflict of interest
There are no conflict of interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Zu is co-first author.
Rights and permissions
About this article
Cite this article
Guo, J., Zu, G., Zhou, T. et al. Clinicopathological significance of orphan nuclear receptor Nurr1 expression in gastric cancer. Clin Transl Oncol 17, 788–794 (2015). https://doi.org/10.1007/s12094-015-1305-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1305-z