Abstract
Human Immunodeficiency Virus (HIV) is a major global healthcare burden. Current lifelong antiretroviral therapy drastically improves life expectancy but do not cure HIV. Therefore, at the existing growth rates, it is estimated that around 42 million people will be living with HIV by 2030 worldwide. A cure for HIV is need of the hour which could come in the form of remission (durable viral control without ART) or eradication (complete removal of latent replication-competent virus). In this review, we discuss recent advances in basic, applied and clinical aspects of latent HIV reservoirs including its tissue locations, cell types, cell properties, genomic integration sites and its significance, mechanism of reservoir seeding and methods to study the reservoirs. Natural models of functional cure which include elite controllers, viremic controllers, long term non-progressors and post-treatment controllers are discussed. Recent advances towards a functional HIV cure are discussed under headings; CCR5Δ32/Δ32 stem cells transplantation, shock and kill strategy, block and lock strategy, gene therapy and combined strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HIV is a retrovirus that transmits through infected blood, semen or vaginal fluids and preferentially destroys CD4 + T cells; compromising cell-mediated immunity and inviting opportunistic infections. Acute HIV stage lasts for few weeks and present with flu-like symptoms. Thereafter, person remains asymptomatic for years until he develops Acquired Immune-Deficiency Syndrome(AIDS). HIV can be detected in blood or oral fluid by antibody-based test (23–90 days after exposure), p24 antigen/antibody-based test (18–45 days after exposure) or nucleic acid test (10–33 days after exposure). No effective vaccine or cure exists for HIV, but Anti-Retroviral therapy (ART) drastically slows disease progression. Various preventive measures includes using condoms during sex, screening blood products, never sharing needles, and HIV prevention medicines such as pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP). Healthcare workers wear protective equipment (including gowns, gloves and eyewear) while performing any invasive procedures involving HIV patient’s blood or body fluids. Recent technologies have shorted HIV treatment frequency from taking traditional ART pills every day orally to once a month or once every two months injection (Cabenuva [1]) and even once in 6 months injection (Sunlenca [2]) following undetectable plasma viral load.
At the existing growth rates, around 42 million people could be HIV positive globally by 2030 [3]. COVID-19 pandemic severely disrupted HIV testing services in 2020 and 2021 [4] and therefore, there are high chances of fuelled HIV transmission from unsuppressed Antiretroviral therapy(ART) naïve PLHIV and re-emergence of AIDS pandemic in post COVID-19 era [5]. Further, Socio-economic condition affects peripheral blood CD4 + cell count among HIV positive adults favoring progression towards AIDS [6, 7]. A cure for HIV is desperately needed to end global HIV/AIDS burden.
The scope of this article includes the evolving mechanisms of HIV latency during anti-retroviral therapy and the concept of functional HIV cure naturally and through experimental interventions (i.e., CCR5Δ32/Δ32 stem cells transplantation, Shock and Kill strategy, Block and Lock strategy, Gene therapy and Combined Strategies); Preventive experimental HIV vaccines is beyond the scope of this article. This article assumes significance as it thoroughly explores the recent advances and presents new concepts in understanding HIV reservoirs and functional cures that could accelerate the development and delivery of an HIV cure. An HIV cure could bring tremendous health, economic and social benefits, for example, it will prevent new infections, overcome uneasiness regarding life-long ART treatment, overcome stigma/discrimination, and will ease financial burden of life-long ART.
The Latent Reservoir of HIV
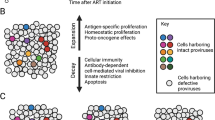
Gut,lymph nodes,spleen,liver and nervous system represents majority of HIV tissue reservoir [8] and cell types includes lymphocytes [9],macrophages [10],dendritic cells [11],astrocytes [12] and adipocytes [13] Fig. 1. HIV is ssRNA virus, however, it gets converted to dsDNA which integrate with host cell DNA Fig. 1. The integrated viral DNA(known as provirus) could remain inactive(latent) for years while the clonal expansion of these provirus harboring cells keep HIV reservoir sustained Fig. 2. Persistence of HIV in these reservoir sites is influenced by cell properties (Response to a specific stimulation, swing from resting state to activated state or vice-versa, transcriptional activity, resistance to immune killing, etc.) and genomic factors (provirus sequence and location, genomic integration site, intact or defective nature of proviruses, etc.). Viral reservoir quantification is usually done by Quantitative Viral Outgrowth Assay [14] and Intact Proviral DNA Assay [15] while genetic characterization of reservoir cells is done by single-cell reservoir profiling techniques(HIV SORT-seq [16]; HIV STIP-seq [17]; ECCITEseq [18]) and full-length individual proviral sequencing.
Mechanism of establishment of HIV latent reservoirs. Following HIV transmission by heterosexual (A1) or homosexual (A2) intercourse with HIV positive partner, mother to child(A3), contaminated blood (A4) or syringe (A5); early initiation of Anti-retroviral drugs drastically controls the plasma viral load and thereby restricting most cells from getting HIV infected (B1). Some cells still get HIV infected and few of these cells are attacked and eliminated by expanded HIV-specific CD8 + T cells (B2). Early initiation of Anti-retroviral drugs together with expanded HIV-specific CD8 + T cells do not allow decline in CD4 + T cells count. Elimination-resistant replication-competent provirus harboring cells get seeded in tissue reservoirs and clonal expansion of these cells keeps the provirus intact for years (B3)
Compartmentalized proviral population with distinct genotype and phenotype are found in some tissues (eg. Brain) [19]. Overall reservoir decay is sluggish with half-life of around 4 years until seven years of ART and around 19 years afterward [20]. The ability for clonal expansion is different for various CD4 + T cell subsets. Effector memory CD4 + cells are enriched in clonally expanded proviral sequences. Macrophages act as distinctive HIV reservoirs. They express CD4 in low levels [21],reside in every tissue,have long-life and are resistant to elimination by CD8 + cells [22] Fig. 2. In urethral tissue, macrophages are reported to harbor integrated proviruses but CD4 + T cells do not [23].
Replication-competent noninduced provirus with intact genomes and normal LTR function within latent reservoir sites is a barrier to HIV cure. Cells harboring provirus do not behave differently from normal cells, therefore are largely invisible to immune system. CD4 + T cells harboring replication-competent provirus are inherently resistant to killing by CD8 + T cells [24] Fig. 2.
Around 2% proviruses remain genetically intact during long-term ART [25]. Majority of proviruses are defective; they can produce HIV transcripts(eg. novel usHIV-RNAs [26]) or HIV proteins(eg. HIV-1 Gag and Nef proteins [27]) resulting persistent immune activation, but cannot produce new progency virus. Defective provirus levels are unaffected during ART [28].
HIV suppression at younger age is linked to smaller HIV DNA level than at higher age [29]. ART started during early stage of HIV infection restricts size of HIV reservoir but do not drastically change proviral landscape [25]. Bulk of proviruses inside viral reservoirs is from latest time point; however,previous proviral variants continue to archive [30]. The extensive diversity of proviral variants within HIV reservoirs challenges HIV eradication or cure. In latently infected cells, the lack of pro-apoptotic viral proteins(HIV Env,protease and Vpr),along with increased host anti-apoptotic(Bcl-2,XIAP) and reduced host pro-apoptotic cell proteins(BAK/Bax),altogether encourage the latent cell survival.
Genomic Sites where virus integrates is critically important in determining the proliferation and persistence of infected cells. HIV integrating within cancer genes encourages expansion of infected cells and slows down virus decay. Provirus containing cells have wider distribution with notable enrichment during cancer metastases [31]. Some integration sites within host genome(eg. KRAB domain-containing ZNF genes for intact proviruses [32]) are more capable to maintain HIV latency in clonally expanded memory CD4 + T cells. Elite controllers have proviruses integration within centromeric satellite DNA which is transcription inactive [33]. Proviruses binding at active transcription sites make HIV infected cells prone to immune elimination.
Natural Models for Functional Cure
Few PLHIVs(Prevalence: 0.5%) are able to sustain undetectable plasma RNA HIV loads for minimum 12 months without ART(Elite controllers). However, around three-fourth of elite controllers experience long-term HIV disease progression and only 17% did not exhibited sign of HIV disease progression after 17 years of follow up [34]. Viremic controllers are similar but bigger subsets of PLHIVs(Prevalence: 3%-4%) who can keep their plasma RNA HIV load suppressed < 2000 copies/ml over a year without ART.
Long term non-progressors are PLHIVs who can maintain CD4 cell counts ≥ 500 cells/μL for ≥ 7 years without ART (Prevalence: 3%-4%). Elite or viremic controllers may classify as long term non-progressors in some cases and vice-versa. Actually, 52% long-term non progressors are reported elite controllers [35]. 30 years survival probability is 0.9 for Elite controllers plus long-term non progressors and 0.7 for viremic controllers plus long-term non progressors [35]. About 55% Long term non-progressors can maintain their status after 20 years but just 14% after 30 years.
Post-treatment controllers are PLHIVs who can suppress viral load ≤ 400 copies/mL post ≥ 24 weeks of ART interruption. Non-controllers usually have a swift viral rebound in 3–4 weeks after ART discontinuation [36]. Control of HIV after Antiretroviral Medication Pause(CHAMP) study determined the prevalence of Post-treatment controllers as 4% when ART initiated during chronic infection and 13% when ART initiated during early infection [37]. However, only 20% Post-treatment controllers had suppressed viral load after ≥ 5years of ART interruption.
Size of HIV reservoir plays an important role in the natural control of HIV infection in absence of ART. Starting ART during early HIV stage drastically restrict the size of HIV reservoir [38]. A restricted viral reservoir can give long-term advantages in terms of preserving functional cell-mediated immunity and a greater window period to viral rebound. Unlike elite controllers where functional capabilities of cytotoxic T cells are not altered over time when integrated viral DNA gradually increases [38],Post-treatment controllers present with enhanced cytotoxic T cells functional capabilities over time [39]. Defective proviruses may not explain reason for viral control among Elite controllers or Viremic controllers rather than it is through mechanism yet not known.
Strategies for Functional Cure (Fig. 3)
CCR5Δ32/Δ32 Stem Cells Transplantation
HIV-1 entry into CD4 + cells is dependent on co-receptors mainly CCR5 and CXCR4. Homozygous 32-base pair deletion in CCR5 allele(CCR5Δ32/Δ32) block cell-surface expression of CCR5 conferring natural resistance to CCR5-tropic HIV variants(R5) [40]. The frequency of CCR5-Δ32 allele ranges from 16.4% in Norwegian hematopoietic stem cell donors to 0% in Ethiopian donors, however, < 1% are homozygous [41, 42]. However, even if full chimerism is achieved post CCR5Δ32/Δ32 stem cells transplant, donor originated CD4 + cells could still be susceptible to another HIV variant(CXCR4-tropic/X4 variant).
So far, Berlin patient (2008) [43], London patient (2019) [44], City of hope patient (2022)[45], New York patient (2022) [46] and Düsseldorf patient (2023) [47] are presumed cured via CCR5Δ32/Δ32 stem cells transplantation. These five cases were given stem cells transplantation primarily for the treatment of their cancers but no HIV-1 rebound following ART disruption is yet reported. HIV is one of the chronic microbial infections that can be found among cancer patients [48, 49]. Other two patients: Boston patients (2014) [50] and Essen patient (2019) [51] reported viral rebound. Berlin patient died 13 years after transplant due to relapse of acute myeloid leukemia but there was no proof of HIV-1 rebound. Unlike other four male patients, New York patient(2022) is a mixed-race middle-aged HIV-1 positive woman who received haplo-cord transplant(CCR5Δ32/Δ32 cord blood cells) for the treatment of acute myeloid leukemia and never developed Graft-versus-host disease [46]. A very close HLA matching of donor and recipient is not required for umbilical cord stem cells transplant unlike haematopoietic stem-cell transplant in other four patients.
Shock and Kill Strategy
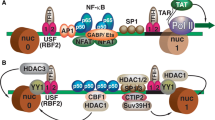
In this strategy, drugs classified as latency-reversing agents(LRAs) induce reactivation of the latent proviral transcription (“shock” component). The reactivated cells and virions produced are then supposed to be eliminated by virus induced cell death, immune mediated clearance or anti HIV drugs(“kill” component). LRAs are classified based on the host factor they act upon to reactivate latent proviruses and include epigenetic modifiers (HDAC inhibitors, HMT inhibitors, DNA methylation inhibitors), PKC agonists (Ingenol derivatives,prostatin,bryostatin-1), Inducers of P-TEFb release (BETis), TLR agonists (Flagellin, Pam3CSK4,GS-9620, MGN1703), SMAC mimetics (Ciapavir, AZD5582), STAT5 sumoylation inhibitors(HOAt and HODHBt),MAPK agonist(Procyanidin trimer C1),CCR5 antagonist(Maraviroc),Tat vaccine(Tat Oyi vaccine,Tat-R5M4 protein),IL-15 agonist(ALT-803), immune checkpoint inhibitors [anti-PD-1(nivolumab,pembrolizumab),anti-CTLA-4(ipilimumab)] and so on [52].
PKC agonists are potent NF-κB activators. Activated NF-κB bind to NF-κB motifs in the enhancer region of HIV-1 LTR to initiate viral transcription in latent cells [53]. HDACi class-I have specificity for latent reservoirs and minimal inference with PKC agonist [54],therefore, HDACi class-I can have a synergistic combination with PKC agonist(shocktail). The concept of prodrugs can be utilized to transport LRAs to HIV reservoirs at peak concentrations with lesser toxicities. LRAs can be tried with early ART at acute HIV stage(rather than suppressive stage) to disturb latency establishment, reduce reservoir size and facilitate HIV elimination.
Even in case of effective viral reactivation, success in eliminating viral reservoir(“kill” component) could be limited by several immunological roadblocks like deficient recognisation, exhaustion, hyperactive cytokines, deficient cytotoxicity, etc. [55,56,57]. Shock and kill strategy risk damage to neurons if latent HIV-1 reservoirs in central nervous system get reactivated by LRAs. Further, it risks expansion of HIV reservoirs if “kill” part is ineffective. Labeling HIV reservoirs with apoptosis inducers [Bcl-2 inhibitors(eg. Venetoclax, Navitoclax), XIAP antagonists, PI3K/Akt antagonists and RIG-I inducers] after LRA activity can selectively eliminate HIV reservoirs.
Block and Lock Strategy
This strategy aims to achieve permanent silencing of proviral latent reservoir following interruption of antiretroviral therapy. “Block” of sporadic proviral reactivation to “lock” integrated provirus into a deep latency can accomplish a long-term durable HIV remission(functional cure).
HIV silencing can be done by (1) RNA-Induced Epigenetic Silencing [eg. siRNA] (2)Tat inhibition[eg. didehydro-cortistatin A(dCA),Tat degradation[eg. Triptolide],CDK9 inhibition[eg. Flavopiridol],transcription factor IIH inhibition[eg. Spironolactone] (3) mTOR inhibition[eg. rapamycin], Akt inhibition[eg. uprosertib], HSP90 inhibition[eg.GV1001] (4) Inhibiting JAK/STAT pathways[eg. filgotinib,ruxolitinib] (5) Calcium sensitization[eg. levosimendan, manidipine] (6) Nucleotide synthesis inhibition[eg. MMF] (7) RNA splicing inhibition[eg. Filgotinib] (8) FACT and RNA Pol II inhibition[eg. curaxin CBL0100] (9) HIV-1 integration site modification[eg. LEDGIN] (10) Trx/TrxR pathway inhibition [eg. tiopronin] (11) BRD4 modulation[eg. ZL0580] [58]
People living with blocked and locked HIV may be fearful for its reactivation. However, fossils of retroviruses are found throughout the human genome(endogenous retroviruses) as they continued to integrate and inherit from millions of years without causing any harm [59]. Block and lock strategy can be combined with shock and kill strategy for a functional cure, although the two look opposite strategies. Since LRAs have limited capability for HIV reactivation at non-toxic concentrations, Shock and kill strategy may first eliminate readily inducible latent reservoirs followed by block and lock strategy to permanently silence the reservoirs which could not reactivated by existing LRAs. Majority of proviruses in latent reservoirs are defective in producing infectious virions, yet they generate viral proteins to cause immune-activation [27, 60]. Block and lock strategy can silence replication-competent as well as replication-defective proviruses. Even a partial silencing can subsequently reduce the reservoir size making it easier to control residual viruses by engineered immune-response.
Gene Therapy
In most Gene therapies, a single-time treatment has life-long curative capability which can be transformative for PLHIVs. Gene therapy could be done ex-vivo or in-vivo [61]. In ex-vivo strategies,CCR5 co-receptor or HIV provirus are deleted/inactivated in autologous CD4 + cells via CRISPR–Cas9 and recombinases technologies; OR Chimeric antigen receptor(CAR) are employed on autologous T cells to recognize HIV envelope proteins and then re-infused into PLHIVs. The former may reduce but not completely eradicate viral reservoirs due to continuous cloning of in-vivo CD4 + cells. Infused CAR T cells expand in-vivo only in presence of adequate viral antigen [62] and viral antigens are too low during ART. Therefore,Analytical treatment interruption or potent LRA or exogenous cell-associated HIV envelope may facilitate the expansion of infused CAR T cells. Progress in cancer CAR T cell therapies can benefit HIV cure therapy. Ex-vivo cell modification in automated closed-system equipments at point-of-care will be needed for application in low-resource settings.
Among in-vivo strategies, AAV-delivered CRISPR/Cas9 gene editing construct injected in PLHIV can excise integrated proviral DNA in reservoir cells at all major anatomical sites. EBT-101 is one such construct that is granted Fast Track designation by US-FDA Table 1. AAV-mediated antibody gene delivery (eg. PG9, a potent anti-HIV bnAb) [63], AAV-delivered eCD4-Ig(CD4 mimetics) [64] or CD4 + cell-homing mRNA-LNPs [65], are other promising in-vivo strategies [66]. Novel techniques utilizing Antibody–drug conjugate could act as a biological missile to target proviral reservoirs [67]. Antibody–drug conjugate is made of monoclonal antibody attached to a cytotoxic drug for highly specific killing of target cells.
Ongoing gene therapy based HIV cure clinical trials are summarized in Table 1. While still at an early-stage,in-vivo gene therapy is a promising strategy for HIV cure. Recognizing approaches to drive down the cost of gene therapies will be required for widespread applicability in low and middle income countries and therefore,Global Gene Therapy Initiative was created in 2020 for this purpose [68].
Combined Strategies
-
Combination Immunotherapies: Broadly neutralizing antibodies 3BNC117 delay viral rebound following ART interruption [69] and therefore are tested with LRAs in Phase IIa TITAN trial and ROADMAP trial [70, 71]. HIVconsv T cell vaccine combined with romidepsin(HDAC inhibitor) can control viremia and reseeding of viral reservoir after ART interruption [72]. HIVconsv vaccine during early ART induce post-treatment controllers like highly functional T-cell responses (RIVER trial) [73]. HIVconsv T cell vaccine may enhance immune-surveillance and long-term control of residual reservoir cells after latency reversal by LRAs and rapidly eliminated by 3BNC117 [74].
-
Block, Lock and Excise: This approach aims to inhibit viral transcription(block),keeping the virus blocked or silenced without ART(lock) and permanently inactivating proviral DNA using epigenomic and genomic approaches(excise). The graded transformation of residual HIV among PLHIVs from latent to silent into permanently defective proviruses is an acceleration of the natural path to endogenous retrovirues. HIV Obstruction by Programmed Epigenetics(HOPE) Collaboratory is working toward this approach for both “functional” and “classical” cure. Their objective include targeted genome engineering to disable HIV provirus by developing double strand break-free genome engineering method(Brec1,dCas9,DNA-PNA) and establishing in-vivo delivery platforms(VLP,PLGA-NP,Brec1–αhuCD7 conjugates) [75].
Concluding Remarks
This study focused on the newer concepts emerging in last few years which could shape HIV cure research. The disadvantage of the study is that it could only provide an overview of emerging concepts in HIV cure research because each sub-section has itself a vast scope to review.
The spectrum of HIV cure research has significantly evolved in the last few years. Four more patients(Düsseldorf patient, City of hope patient, New York patient and London patient) reported HIV remission and possible cure through CCR5Δ32/Δ32 stem cells transplantation in last five years after Berlin patient(2009). A Few LRAs have remarkably succeeded in reversing HIV latency in human trials. Addition of bNAbs and therapeutic vaccines to these LRAs has cured primate models enhancing hope of success in undergoing clinical trials. A bNAbs(3BNC117) changed the course of HIV disease and diminished reservoir size when administrated at start of ART. Novel interventions like epigenetic modification to block proviral activation,AAV-delivered CRISPR gene-editing to delete or permanently inactivate proviruses,AAV-delivered CD4 mimetics that bind viral envelope and neutralize all HIV subtypes,engineering autologous CD4 + cells to block viral entry,engineered B cells that express HIV specific bNAbs,drugs to sensitize reservoir cells towards apoptosis and drug-delivery platforms to viral reservoirs are promising approaches that are currently under active investigation and can change the direction of HIV cure research. Due to limitations associated with complete eradication of HIV,a durable control of viral replication without ART is a more achievable aim of HIV cure research community among in-vivo trials.
Low-income high-burden countries have genetically and biologically diverse proviral population with geographically and ethnically diversities in host immunity. Recently established HIV Cure Africa Acceleration Partnership could enable wider engagement. People from multiple academia,industries,private sector,community and government are coming together to pool common resources and coordinate complex studies with only aim of expediting research for a globally applicable,acceptable,and affordable HIV cure. There is no limitation of funds and field is highly committed to bring up an effective and scalable remission or cure.
References
Taki E, Soleimani F, Asadi A, Ghahramanpour H, Namvar A, Heidary M (2022) Cabotegravir/Rilpivirine: the last FDA-approved drug to treat HIV. Expert Rev Anti Infect Ther 20:1135–1147. https://doi.org/10.1080/14787210.2022.2081153
Prather C, Lee A, Yen C (2023) Lenacapavir: A first-in-class capsid inhibitor for the treatment of highly treatment-resistant HIV. Am J Health Syst Pharm 80:1774–1780. https://doi.org/10.1093/ajhp/zxad223
Dybul M, Attoye T, Baptiste S, Cherutich P, Dabis F, Deeks SG, Dieffenbach C, Doehle B, Goodenow MM, Jiang A, Kemps D, Lewin SR, Lumpkin MM, Mathae L, McCune JM, Ndung’u T, Nsubuga M, Peay HL, Pottage J, Warren M, Sikazwe I (2021) Sunnylands 2019 working group. The case for an HIV cure and how to get there. Lancet HIV 8:e51–e58. https://doi.org/10.1016/S2352-3018(20)30232-0
Maurya SP, Sharma A, Singh R, Gautam H, Das BK (2022) HIV testing & diagnosis in 2020 at the apex tertiary referral hospital of India: impact of COVID-19 pandemic. AIDS Care 34:828–831. https://doi.org/10.1080/09540121.2021.1975631
Thankur P et al (2024) Post COVID-19 era: Re-emergence of known and future (X) viral pathogens. Vacunas. https://doi.org/10.1016/j.vacun.2024.02.001
Maurya SP, Singh R, Negi N, Vajpayee M, Kapil A, Das BK (2020) The impact of education, family income, and occupation on CD4 count among HIV infected adults. Indian J Sex Transm Dis AIDS 41:130–131. https://doi.org/10.4103/ijstd.IJSTD_11_17
Maurya SP, Singh R, Negi N, Kapil A, Chaudhry R, Das BK (2016) The level of education affects CD4 cell count and wellness among HIV infected adult between age group 18 to 60 years. Int J Infect Dis 45:264. https://doi.org/10.1016/j.ijid.2016.02.589
Wong JK, Yukl SA (2016) Tissue reservoirs of HIV. Curr Opin HIV AIDS 11:362–370. https://doi.org/10.1097/COH.0000000000000293
Sengupta S, Siliciano RF (2018) Targeting the latent reservoir for HIV-1. Immunity 48:872–895. https://doi.org/10.1016/j.immuni.2018.04.030
Veenhuis RT, Abreu CM, Costa PAG, Ferreira EA, Ratliff J, Pohlenz L, Shirk EN, Rubin LH, Blankson JN, Gama L, Clements JE (2023) Monocyte-derived macrophages contain persistent latent HIV reservoirs. Nat Microbiol 8:833–844. https://doi.org/10.1038/s41564-023-01349-3
Banga R, Procopio FA, Lana E, Gladkov GT, Roseto I, Parsons EM, Lian X, Armani-Tourret M, Bellefroid M, Gao C, Kauzlaric A, Foglierini M, Alfageme-Abello O, Sluka SHM, Munoz O, Mastrangelo A, Fenwick C, Muller Y, Mkindi CG, Daubenberger C, Cavassini M, Trunfio R, Déglise S, Corpataux JM, Delorenzi M, Lichterfeld M, Pantaleo G, Perreau M (2023) Lymph node dendritic cells harbor inducible replication-competent HIV despite years of suppressive ART. Cell Host Microbe 31:1714-1731.e9. https://doi.org/10.1016/j.chom.2023.08.020
Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ (2003) Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol 13:144–154. https://doi.org/10.1111/j.1750-3639.2003.tb00014.x
Damouche A, Lazure T, Avettand-Fènoël V, Huot N, Dejucq-Rainsford N, Satie AP, Mélard A, David L, Gommet C, Ghosn J, Noel N, Pourcher G, Martinez V, Benoist S, Béréziat V, Cosma A, Favier B, Vaslin B, Rouzioux C, Capeau J, Müller-Trutwin M, Dereuddre-Bosquet N, Le Grand R, Lambotte O, Bourgeois C (2015) Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog 11:e1005153. https://doi.org/10.1371/journal.ppat.1005153
Laird GM, Eisele EE, Rabi SA, Lai J, Chioma S, Blankson JN, Siliciano JD, Siliciano RF (2013) Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog 9:e1003398. https://doi.org/10.1371/journal.ppat.1003398
Bruner KM, Wang Z, Simonetti FR, Bender AM, Kwon KJ, Sengupta S, Fray EJ, Beg SA, Antar AAR, Jenike KM, Bertagnolli LN, Capoferri AA, Kufera JT, Timmons A, Nobles C, Gregg J, Wada N, Ho YC, Zhang H, Margolick JB, Blankson JN, Deeks SG, Bushman FD, Siliciano JD, Laird GM, Siliciano RF (2019) A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 566:120–125. https://doi.org/10.1038/s41586-019-0898-8
Liu R, Yeh YJ, Varabyou A, Collora JA, Sherrill-Mix S, Talbot CC Jr, Mehta S, Albrecht K, Hao H, Zhang H, Pollack RA, Beg SA, Calvi RM, Hu J, Durand CM, Ambinder RF, Hoh R, Deeks SG, Chiarella J, Spudich S, Douek DC, Bushman FD, Pertea M, Ho YC (2020) Single-cell transcriptional landscapes reveal HIV-1-driven aberrant host gene transcription as a potential therapeutic target. Sci Transl Med 12:eaaz0802. https://doi.org/10.1126/scitranslmed.aaz0802
Cole B, Lambrechts L, Gantner P, Noppe Y, Bonine N, Witkowski W, Chen L, Palmer S, Mullins JI, Chomont N, Pardons M, Vandekerckhove L (2021) In-depth single-cell analysis of translation-competent HIV-1 reservoirs identifies cellular sources of plasma viremia. Nat Commun 12:3727. https://doi.org/10.1038/s41467-021-24080-1
Collora JA, Liu R, Pinto-Santini D, Ravindra N, Ganoza C, Lama JR, Alfaro R, Chiarella J, Spudich S, Mounzer K, Tebas P, Montaner LJ, van Dijk D, Duerr A, Ho YC (2022) Single-cell multiomics reveals persistence of HIV-1 in expanded cytotoxic T cell clones. Immunity 55:1013-1031.e7. https://doi.org/10.1016/j.immuni.2022.03.004
Wang C, Schlub TE, Yu WH, Tan CS, Stefic K, Gianella S, Smith DM, Lauffenburger DA, Chaillon A, Julg B (2022) Landscape of human immunodeficiency virus neutralization susceptibilities across tissue reservoirs. Clin Infect Dis 75:1342–1350. https://doi.org/10.1093/cid/ciac164
Peluso MJ, Bacchetti P, Ritter KD, Beg S, Lai J, Martin JN, Hunt PW, Henrich TJ, Siliciano JD, Siliciano RF, Laird GM, Deeks SG (2020) Differential decay of intact and defective proviral DNA in HIV-1-infected individuals on suppressive antiretroviral therapy. JCI Insight 5:e132997. https://doi.org/10.1172/jci.insight.132997
Zhen A, Krutzik SR, Levin BR, Kasparian S, Zack JA, Kitchen SG (2014) CD4 ligation on human blood monocytes triggers macrophage differentiation and enhances HIV infection. J Virol 88:9934–9946. https://doi.org/10.1128/JVI.00616-14
Clayton KL, Collins DR, Lengieza J, Ghebremichael M, Dotiwala F, Lieberman J, Walker BD (2018) Resistance of HIV-infected macrophages to CD8+ T lymphocyte-mediated killing drives activation of the immune system. Nat Immunol 19:475–486. https://doi.org/10.1038/s41590-018-0085-3
Ganor Y, Real F, Sennepin A, Dutertre CA, Prevedel L, Xu L, Tudor D, Charmeteau B, Couedel-Courteille A, Marion S, Zenak AR, Jourdain JP, Zhou Z, Schmitt A, Capron C, Eugenin EA, Cheynier R, Revol M, Cristofari S, Hosmalin A, Bomsel M (2019) HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat Microbiol 4:633–644. https://doi.org/10.1038/s41564-018-0335-z
Huang SH, Ren Y, Thomas AS, Chan D, Mueller S, Ward AR, Patel S, Bollard CM, Cruz CR, Karandish S, Truong R, Macedo AB, Bosque A, Kovacs C, Benko E, Piechocka-Trocha A, Wong H, Jeng E, Nixon DF, Ho YC, Siliciano RF, Walker BD, Jones RB (2018) Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J Clin Invest 128:876–889. https://doi.org/10.1172/JCI97555
Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, Lai J, Strain MC, Lada SM, Hoh R, Ho YC, Richman DD, Deeks SG, Siliciano JD, Siliciano RF (2016) Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med 22:1043–1049. https://doi.org/10.1038/nm.4156
Imamichi H, Dewar RL, Adelsberger JW, Rehm CA, O’Doherty U, Paxinos EE, Fauci AS, Lane HC (2016) Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci USA 113:8783–8788. https://doi.org/10.1073/pnas.1609057113
Imamichi H, Smith M, Adelsberger JW, Izumi T, Scrimieri F, Sherman BT, Rehm CA, Imamichi T, Pau A, Catalfamo M, Fauci AS, Lane HC (2020) Defective HIV-1 proviruses produce viral proteins. Proc Natl Acad Sci USA 117:3704–3710. https://doi.org/10.1073/pnas.1917876117
Gandhi RT, Cyktor JC, Bosch RJ, Mar H, Laird GM, Martin A, Collier AC, Riddler SA, Macatangay BJ, Rinaldo CR, Eron JJ, Siliciano JD, McMahon DK, Mellors JW (2021) AIDS clinical trials group A5321 team. Selective decay of intact HIV-1 proviral DNA on antiretroviral therapy. J Infect Dis 223:225–233
Golob JL, Stern J, Holte S, Kitahata MM, Crane HM, Coombs RW, Goecker E, Woolfrey AE, Harrington RD (2018) HIV DNA levels and decay in a cohort of 111 long-term virally suppressed patients. AIDS 32:2113–2118. https://doi.org/10.1097/QAD.0000000000001948
Brooks K, Jones BR, Dilernia DA, Wilkins DJ, Claiborne DT, McInally S, Gilmour J, Kilembe W, Joy JB, Allen SA, Brumme ZL, Hunter E (2020) HIV-1 variants are archived throughout infection and persist in the reservoir. PLoS Pathog 16:e1008378. https://doi.org/10.1371/journal.ppat.1008378
Simonetti FR, Sobolewski MD, Fyne E, Shao W, Spindler J, Hattori J, Anderson EM, Watters SA, Hill S, Wu X, Wells D, Su L, Luke BT, Halvas EK, Besson G, Penrose KJ, Yang Z, Kwan RW, Van Waes C, Uldrick T, Citrin DE, Kovacs J, Polis MA, Rehm CA, Gorelick R, Piatak M, Keele BF, Kearney MF, Coffin JM, Hughes SH, Mellors JW, Maldarelli F (2016) Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci USA 113:1883–1888. https://doi.org/10.1073/pnas.1522675113
Huang AS, Ramos V, Oliveira TY, Gaebler C, Jankovic M, Nussenzweig MC, Cohn LB (2021) Integration features of intact latent HIV-1 in CD4+ T cell clones contribute to viral persistence. J Exp Med 218:e20211427
Jiang C, Lian X, Gao C, Sun X, Einkauf KB, Chevalier JM, Chen SMY, Hua S, Rhee B, Chang K, Blackmer JE, Osborn M, Peluso MJ, Hoh R, Somsouk M, Milush J, Bertagnolli LN, Sweet SE, Varriale JA, Burbelo PD, Chun TW, Laird GM, Serrao E, Engelman AN, Carrington M, Siliciano RF, Siliciano JM, Deeks SG, Walker BD, Lichterfeld M, Yu XG (2020) Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 585:261–267. https://doi.org/10.1038/s41586-020-2651-8
Borrell M, Fernández I, Etcheverrry F, Ugarte A, Plana M, Leal L, García F (2021) High rates of long-term progression in HIV-1-positive elite controllers. J Int AIDS Soc 24:e25675. https://doi.org/10.1002/jia2.25675
Capa L, Ayala-Suárez R, De La Torre Tarazona HE, González-García J, Del Romero J, Alcamí J, Díez-Fuertes F (2022) Elite controllers long-term non progressors present improved survival and slower disease progression. Sci Rep 12:16356. https://doi.org/10.1038/s41598-022-19970-3
Li JZ, Etemad B, Ahmed H, Aga E, Bosch RJ, Mellors JW, Kuritzkes DR, Lederman MM, Para M, Gandhi RT (2016) The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 30:343–353. https://doi.org/10.1097/QAD.0000000000000953
Namazi G, Fajnzylber JM, Aga E, Bosch RJ, Acosta EP, Sharaf R, Hartogensis W, Jacobson JM, Connick E, Volberding P, Skiest D, Margolis D, Sneller MC, Little SJ, Gianella S, Smith DM, Kuritzkes DR, Gulick RM, Mellors JW, Mehraj V, Gandhi RT, Mitsuyasu R, Schooley RT, Henry K, Tebas P, Deeks SG, Chun TW, Collier AC, Routy JP, Hecht FM, Walker BD, Li JZ (2018) The control of HIV after antiretroviral medication pause(CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis 218:1954–1963. https://doi.org/10.1093/infdis/jiy479
Pinzone MR, Graf E, Lynch L, McLaughlin B, Hecht FM, Connors M, Migueles SA, Hwang WT, Nunnari G, O’Doherty U (2016) Monitoring integration over time supports a role for cytotoxic T lymphocytes and ongoing replication as determinants of reservoir size. J Virol 90:10436–10445. https://doi.org/10.1128/JVI.00242-16
Streeck H, Jessen H, Alter G, Teigen N, Waring MT, Jessen A, Stahmer I, van Lunzen J, Lichterfeld M, Gao X, Allen TM, Carrington M, Walker BD, Rockstroh JK, Altfeld M (2006) Immunological and virological impact of highly active antiretroviral therapy initiated during acute HIV-1 infection. J Infect Dis 194:734–739. https://doi.org/10.1086/503811
Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien SJ (1996) Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia growth and development study, multicenter AIDS cohort study, multicenter hemophilia cohort study, San Francisco City cohort. ALIVE Study Sci 273:1856–1862. https://doi.org/10.1126/science.273.5283.1856
Solloch UV, Lang K, Lange V, Böhme I, Schmidt AH, Sauter J (2017) Frequencies of gene variant CCR5-Δ32 in 87 countries based on next-generation sequencing of 13 million individuals sampled from 3 national DKMS donor centers. Hum Immunol 78:710–717. https://doi.org/10.1016/j.humimm.2017.10.001
Martinson JJ, Chapman NH, Rees DC, Liu YT, Clegg JB (1997) Global distribution of the CCR5 gene 32-basepair deletion. Nat Genet 16:100–103. https://doi.org/10.1038/ng0597-100
Brown TR (2015) I am the Berlin patient: a personal reflection. AIDS Res Hum Retroviruses 31:2–3. https://doi.org/10.1089/AID.2014.0224
Gupta RK, Peppa D, Hill AL, Gálvez C, Salgado M, Pace M, McCoy LE, Griffith SA, Thornhill J, Alrubayyi A, Huyveneers LEP, Nastouli E, Grant P, Edwards SG, Innes AJ, Frater J, Nijhuis M, Wensing AMJ, Martinez-Picado J, Olavarria E (2020) Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV 7:e340–e347. https://doi.org/10.1016/S2352-3018(20)30069-2
Dickter JK, Aribi A, Cardoso AA, Gianella S, Gendzekhadze K, Li S, Feng Y, Chaillon A, Laird GM, Browning DL, Ross JA, Nanayakkara DD, Puing A, Stan R, Lai LL, Chang S, Kidambi TD, Thomas S, Al Malki MM, Nakamura R, Alvarnas J, Taplitz RA, Dadwal SS, Forman SJ, Zaia JA (2024) HIV-1 remission after allogeneic hematopoietic-cell transplantation. N Engl J Med 390:669–671. https://doi.org/10.1056/NEJMc2312556
Hsu J, Van Besien K, Glesby MJ, Pahwa S, Coletti A, Warshaw MG, Petz L, Moore TB, Chen YH, Pallikkuth S, Dhummakupt A, Cortado R, Golner A, Bone F, Baldo M, Riches M, Mellors JW, Tobin NH, Browning R, Persaud D, Bryson Y (2023) International maternal pediatric adolescent AIDS clinical trials network(IMPAACT) P1107. Team HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 186:1115-1126.e8. https://doi.org/10.1016/j.cell.2023.02.030
Jensen BO, Knops E, Cords L, Lübke N, Salgado M, Busman-Sahay K, Estes JD, Huyveneers LEP, Perdomo-Celis F, Wittner M, Gálvez C, Mummert C, Passaes C, Eberhard JM, Münk C, Hauber I, Hauber J, Heger E, De Clercq J, Vandekerckhove L, Bergmann S, Dunay GA, Klein F, Häussinger D, Fischer JC, Nachtkamp K, Timm J, Kaiser R, Harrer T, Luedde T, Nijhuis M, Sáez-Cirión A, Schulze Zur Wiesch J, Wensing AMJ, Martinez-Picado J, Kobbe G (2023) In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat Med 29:583–587. https://doi.org/10.1038/s41591-023-02213-x
Yarchoan R, Uldrick TS (2018) HIV-associated cancers and related diseases. N Engl J Med 378:1029–1041. https://doi.org/10.1056/NEJMra1615896
Maurya SP (2011) Streptococcus bovis bacteremia and colorectal carcinoma. Indian J Cancer 48:375–376. https://doi.org/10.4103/0019-509X.84924
Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, Robles YP, Davis BT, Li JZ, Heisey A, Hill AL, Busch MP, Armand P, Soiffer RJ, Altfeld M, Kuritzkes DR (2014) Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med 161:319–327. https://doi.org/10.7326/M14-1027
Verheyen J, Thielen A, Lübke N, Dirks M, Widera M, Dittmer U, Kordelas L, Däumer M, de Jong DCM, Wensing AMJ, Kaiser R, Nijhuis M, Esser S (2019) Rapid rebound of a preexisting CXCR4-tropic human immunodeficiency virus variant after allogeneic transplantation with CCR5 Δ32 homozygous stem cells. Clin Infect Dis 68:684–687. https://doi.org/10.1093/cid/ciy565
Kim Y, Anderson JL, Lewin SR (2018) Getting the “Kill” into “shock and kill”: strategies to eliminate latent HIV. Cell Host Microbe 23:14–26. https://doi.org/10.1016/j.chom.2017.12.004
Bhange D, Prasad N, Singh S, Prajapati HK, Maurya SP, Gopalan BP, Nadig S, Chaturbhuj D, Jayaseelan B, Dinesha TR, Ahamed SF, Singh N, Brahmaiah A, Mehta K, Gohil Y, Balakrishnan P, Das BK, Dias M, Gangakhedkar R, Mehendale S, Paranjape RS, Saravanan S, Shet A, Solomon SS, Thakar M, Ranga U (2021) The evolution of regulatory elements in the emerging promoter-variant strains of HIV-1 subtype C. Front Microbiol 16:779472. https://doi.org/10.3389/fmicb.2021.779472
Lopes JR, Chiba DE, Dos Santos JL (2021) HIV latency reversal agents: a potential path for functional cure? Eur J Med Chem 5:113213. https://doi.org/10.1016/j.ejmech.2021.113213
Bashiri K, Rezaei N, Nasi M, Cossarizza A (2018) The role of latency reversal agents in the cure of HIV: a review of current data. Immunol Lett 196:135–139. https://doi.org/10.1016/j.imlet.2018.02.004
Singh R, Maurya SP, Das N, Kabra SK, Lodha R, Das BK (2022) Immunological factors associated with discordant virological response postcombination antiretroviral therapy in pediatric human immunodeficiency virus infection. Indian J Pharmacol 54:278–281. https://doi.org/10.4103/ijp.ijp_616_21
Maurya SP, Das BK, Singh R, Tyagi S (2019) Effect of Withania somnifer on CD38 expression on CD8+ T lymphocytes among patients of HIV infection. Clin Immunol 203:122–124. https://doi.org/10.1016/j.clim.2019.04.003
Moranguinho I, Valente ST (2020) Block-and-lock: new horizons for a cure for HIV-1. Viruses 12:1443. https://doi.org/10.3390/v12121443
Jern P, Coffin JM (2008) Effects of retroviruses on host genome function. Annu Rev Genet 42:709–732. https://doi.org/10.1146/annurev.genet.42.110807.091501
Paiardini M, Müller-Trutwin M (2013) HIV-associated chronic immune activation. Immunol Rev 254:78–101. https://doi.org/10.1111/imr.12079
Kitawi R, Ledger S, Kelleher AD, Ahlenstiel CL (2024) Advances in HIV gene therapy. Int J Mol Sci 25:2771. https://doi.org/10.3390/ijms25052771
Herzig E, Kim KC, Packard TA, Vardi N, Schwarzer R, Gramatica A, Deeks SG, Williams SR, Landgraf K, Killeen N, Martin DW, Weinberger LS, Greene WC (2019) Attacking latent HIV with convertibleCAR-T Cells, a highly adaptable killing platform. Cell 179:880-894.e10. https://doi.org/10.1016/j.cell.2019.10.002
Priddy FH, Lewis DJM, Gelderblom HC, Hassanin H, Streatfield C, LaBranche C, Hare J, Cox JH, Dally L, Bendel D, Montefiori D, Sayeed E, Ackland J, Gilmour J, Schnepp BC, Wright JF, Johnson P (2019) Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV 6:e230–e239. https://doi.org/10.1016/S2352-3018(19)30003-7
Gardner MR, Fellinger CH, Kattenhorn LM, Davis-Gardner ME, Weber JA, Alfant B, Zhou AS, Prasad NR, Kondur HR, Newton WA, Weisgrau KL, Rakasz EG, Lifson JD, Gao G, Schultz-Darken N, Farzan M (2019) AAV-delivered eCD4-Ig protects rhesus macaques from high-dose SIVmac239 challenges. Sci Transl Med 11:5409. https://doi.org/10.1126/scitranslmed.aau5409
Tombácz I, Laczkó D, Shahnawaz H, Muramatsu H, Natesan A, Yadegari A, Papp TE, Alameh MG, Shuvaev V, Mui BL, Tam YK, Muzykantov V, Pardi N, Weissman D, Parhiz H (2021) Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol Ther 29:3293–3304. https://doi.org/10.1016/j.ymthe.2021.06.004
Pandey S, Gupta S, Bharadwaj A et al (2024) Microfluidic systems: recent advances in chronic disease diagnosis and their therapeutic management. Indian J Microbiol. https://doi.org/10.1007/s12088-024-01296-5
Fu Z, Li S, Han S, Shi C, Zhang Y (2022) Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther 7:93. https://doi.org/10.1038/s41392-022-00947-7
Adair JE, Androski L, Bayigga L, Bazira D, Brandon E, Dee L, Deeks S, Draz M, Dubé K, Dybul M, Gurkan U, Harlow E, Kityo C, Louella M, Malik P, Mathews V, McKemey A, Mugerwa H, Muyanja D, Olayiwola O, Orentas RJ, Popovski A, Sheehy J, Ssali F, Nsubuga MS, Tisdale JF, Verhoeyen E, Dropulić B (2023) Towards access for all: 1st working group report for the global gene therapy initiative(GGTI). Gene Ther 30:216–221. https://doi.org/10.1038/s41434-021-00284-4
Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, Shimeliovich I, Patel R, Burke L, Cohen YZ, Hadrigan S, Settler A, Witmer-Pack M, West AP Jr, Juelg B, Keler T, Hawthorne T, Zingman B, Gulick RM, Pfeifer N, Learn GH, Seaman MS, Bjorkman PJ, Klein F, Schlesinger SJ, Walker BD, Hahn BH, Nussenzweig MC, Caskey M (2016) HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535:556–560. https://doi.org/10.1038/nature18929
Gunst JD, Højen JF, Pahus MH, Rosás-Umbert M, Stiksrud B, McMahon JH, Denton PW, Nielsen H, Johansen IS, Benfield T, Leth S, Gerstoft J, Østergaard L, Schleimann MH, Olesen R, Støvring H, Vibholm L, Weis N, Dyrhol-Riise AM, Pedersen KBH, Lau JSY, Copertino DC Jr, Linden N, Huynh TT, Ramos V, Jones RB, Lewin SR, Tolstrup M, Rasmussen TA, Nussenzweig MC, Caskey M, Reikvam DH, Søgaard OS (2023) Impact of a TLR9 agonist and broadly neutralizing antibodies on HIV-1 persistence: the randomized phase 2a TITAN trial. Nat Med 29:2547–2558. https://doi.org/10.1038/s41591-023-02547-6
Gruell H, Gunst JD, Cohen YZ, Pahus MH, Malin JJ, Platten M, Millard KG, Tolstrup M, Jones RB, Conce Alberto WD, Lorenzi JCC, Oliveira TY, Kümmerle T, Suárez I, Unson-O’Brien C, Nogueira L, Olesen R, Østergaard L, Nielsen H, Lehmann C, Nussenzweig MC, Fätkenheuer G, Klein F, Caskey M, Søgaard OS (2022) Effect of 3BNC117 and romidepsin on the HIV-1 reservoir in people taking suppressive antiretroviral therapy(ROADMAP): a randomised, open-label, phase 2A trial. Lancet Microb 3:e203–e214. https://doi.org/10.1016/S2666-5247(21)00239-1
Mothe B, Rosás-Umbert M, Coll P, Manzardo C, Puertas MC, Morón-López S, Llano A, Miranda C, Cedeño S, López M, Alarcón-Soto Y, Melis GG, Langohr K, Barriocanal AM, Toro J, Ruiz I, Rovira C, Carrillo A, Meulbroek M, Crook A, Wee EG, Miró JM, Clotet B, Valle M, Martinez-Picado J, Hanke T, Brander C, Moltó J (2020) BCN02 study investigators HIVconsv vaccines and romidepsin in early-treated. HIV-1-infected individuals: safety, immunogenicity and effect on the viral reservoir(Study BCN02). Front Immunol 6:823. https://doi.org/10.3389/fimmu.2020.00823
Kopycinski J, Yang H, Hancock G, Pace M, Kim E, Frater J, Stöhr W, Hanke T, Fidler S, Dorrell L (2023) RIVER trial study group. Therapeutic vaccination following early antiretr oviral therapy elicits highly functional T cell responses against conserved HIV-1 regions. Sci Rep 13:17155. https://doi.org/10.1038/s41598-023-42888-3
Devarapalli P, Kumari P, Soni S, Mishra V, Yadav S (2022) Patent intelligence of RNA viruses: implications for combating emerging and re-emerging RNA virus based infectious diseases. Int J Biol Macromol 31:1208–1215. https://doi.org/10.1016/j.ijbiomac.2022.08.16
Ndhlovu L. Block, lock,& excise. In: New Strategies in HIV cure. In: AIDS2022: 24th International AIDS Conference,Montreal. July 29- August 2 2022
Acknowledgements
We are thankful to Department of Health Research, Ministry of Health & Family welfare, Government of India for providing fellowship to Dr. Shesh Prakash Maurya under DHR-HRD scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maurya, S.P., Shrivastav, A., Rawat, V.S. et al. HIV Cure: How Far We Have Come?. Indian J Microbiol (2024). https://doi.org/10.1007/s12088-024-01353-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12088-024-01353-z