Abstract
Chikungunya virus (CHIKV) is a re-emerging mosquito-borne alphavirus that poses a threat to human worldwide. Driven by the lack of approved medication and vaccination, research on anti-Chikungunya agents has received great attention. In an effort to determine potential inhibitor of CHIKV, this study aimed at investigating the potential anti-viral activity of Oroxylum indicum extracts towards CHIKV-infected Vero cells. The virucidal, pre- and post-treatment effects of O. indicum were evaluated, using the maximum non-toxic dose of O. indicum methanol and aqueous extracts as determined by cytotoxicity assay. The viral inhibitory effect was assessed by the morphological changes of Vero cells and further confirmed by plaque assay. Both methanol and aqueous extracts of O. indicum had similar cytotoxicity in Vero cells. Interestingly, the virucidal effect of O. indicum aqueous extract revealed a significant reduction on the viral titre (p < 0.05). The prophylactic effect of aqueous extract was demonstrated when the pre-treated cells exhibited a significant anti-CHIKV activity (p < 0.05). However, methanol extract of this plant exerted an anti-viral activity against CHIKV only to a certain extent. Therefore, the aqueous extract of this plant has a potential to inhibit the virus and acts as prophylactic agent against CHIKV. Further studies however are needed to substantiate the finding and to determine the important compound of this plant as well as the mechanism of action in treating CHIKV infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CHIKV was firstly isolated from the serum of a febrile patient in Tanzania in 1953 [1]. Since the discovery, the transmission has been recorded in over 40 countries spanning Africa, Asia and recently in Europe [2]. The geographical spread to other nations was associated with a new clade of CHIKV that was efficiently transmitted by Aedes albopictus mosquitoes [3]. Infection with CHIKV results in fever, headache, rash and polyarthralgias [4]. Despite the CHIKV medical threat, there is no specific treatment and vaccine available in combating this infection. Medicinal plants have been used by people from various cultures around the world as a source of medicine. They are preferred and accepted as a complementary and alternative medicine due to their expected low side effects, inexpensive and high sustainability in nature. Oroxylum indicum is a rare and threatened medicinal plant that is widely grown in Southeast Asia, India and China [5]. In Malaysia, the young leaves of this plant are eaten as vegetable and known as beko, bonglai or kulai among Malaysian local citizens. Oroxylum indicum can be used as a remedy for individuals with rheumatic pain, ulcer, bronchitis and splenomegaly [6]. In addition, it has a good antioxidant, hepatoprotective ability [7] and has been documented to exhibit high free radical scavenging activity [8]. However, to the best of our knowledge, there is a lack of studies conducted on the anti-viral effect of this plant. Therefore, this present study was undertaken to investigate the possible anti-viral properties of O. indicum on CHIKV infection.
Materials and Methods
Preparation of Methanol and Aqueous Extracts
Oroxylum indicum leaves were properly cleaned and washed with distilled water. They were air-dried before being dried in 50 °C incubator and homogenised into a fine powder form. The methanol extraction was done using soxhlet apparatus until the color of methanol solvent appeared to be clear. Subsequently, the methanol extract was concentrated using a vacuum evaporator and left to dry in a fume hood. The modified aqueous extraction was to reflect the traditional use of O. indicum. A total of 450 g leaves were blended with 1 L distilled water. Subsequently, the suspension was filtered using gauze. The residue was discarded and the extract was left in the – 20 °C freezer for a few days before being freeze dried. Finally, the extracts were kept at 4 °C for long-term storage.
Cell Lines and Culture Conditions
Vero cells were maintained in DMEM media supplemented with 5% Fetal Bovine Serum (FBS), 1 Molar 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 1% penicillin-streptomycin in 37 °C with 5% CO2 incubator.
CHIKV Stock
The virus stock was propagated by adding 300 μL of CHIKV stock and 3 mL 1% FBS to 70% confluent Vero cells in a flask. After 1.5 h incubation, the media was discarded and washed once with 1× PBS. Finally, 12 mL of DMEM with 1% FBS was added and the cytopathic effect (CPE) presentations were monitored for 3 days. On the collection day, the supernatants were collected and centrifuged for 10 min at 1500 rpm. The supernatants were aliquoted in 1.5 mL microcentrifuge tube and kept at – 80 °C. The titre of the virus stock was determined by using plaque assay.
Determination of Maximum Non-toxic Dose (MNTD) and 50% Cytotoxic Concentration (CC50) Value
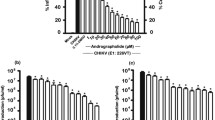
Cytotoxicity assay was carried out on the plant extracts to determine the MNTD and CC50 on Vero cells. MNTD is defined as the highest concentration of extract that would not induce cell death (similar to negative control). CC50 value was expressed as the concentration of extracts that would cause 50% cell death. The cells were seeded at a density of 2 × 104 cells per well in 96 well plate and incubated for 24 h to allow cellular attachment. Two-fold serial dilutions of O. indicum extracts ranging from 10 000 to 39 μg/mL were freshly prepared in growth media supplemented with 2% FBS and 100 μL from each concentration were placed in triplicates. The cell viability was assessed using the CellTiter 96® AQueous one solution cell proliferation assay (Promega). After 48 h, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-5-(3 carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent were pipetted into each well and incubated for 37 °C for 3 h in 5% CO2 incubator. The absorbance was read using ELISA plate reader at 490 nm. MNTD and CC50 value were determined by plotting a graph of the concentration of extract (μg/mL) against percentage of viable cells (Fig. 1). The percentage of viable cells was calculated as: Survival (%) = (T/C) × 100 where C is the mean absorbance of healthy negative control without the extract and T is the mean of absorbance in treated wells with different extract concentration.
Multiplicity of Infection (MOI)
A total of 2 × 104 cells per well were seeded into 96-well plate and incubated overnight. The monolayer of cells were infected with MOI of 0.05, 0.1, 0.5 and 1.0. Virus dilution with different MOIs in 1% FBS was incubated in 37 °C with 5% CO2 incubator. After 1.5 h, the virus was removed and the plate was washed with 1x PBS. Hundred microlitre of 1% FBS was added and incubated at 37 °C in 5% CO2 incubator. The supernatant from each MOI was collected at 0 to 72 h after infection.
Anti-viral Activity
Morphological Changes
The interaction between the O. indicum extracts and CHIKV were initially investigated by the changes of cell morphology and the presence of CPE. The degree of CPE manifestation was characterised according to Tang et al. [9] with slight modifications; (i) ‘−’ for no CPE presentation, (ii) ‘+/−’ for ≤ 10% CPE presentation, (iii) ‘+’ for ≤ 25% CPE presentation, (iv) ‘++’ for ≤ 50% CPE presentation, (v) ‘+++’ for ≤ 75% CPE presentation and (vi) ‘++++’ for 100% CPE presentation.
Virucidal Effect
The virucidal effect of O. indicum extracts on CHIKV was evaluated by incubating CHIKV suspension with the plant extracts for 2 h at 37 °C with 5% CO2 incubator. Then, the titre of the virus was directly measured using plaque assay.
Pre-treatment of Cells with O. indicum Extracts Before CHIKV Infection
Different extract concentrations were added to the cells for 24 h. The cells were gently washed with PBS once to remove the remaining extract inside the wells. Subsequently, CHIKV at MOI of 0.1 was introduced for 1.5 h and the cells were washed with PBS. Next, media with 1% FBS was added into each well and the supernatants were harvested after 24 h post-infection. The morphological changes of the cells were observed and the supernatants were collected to determine the viral titre.
Post-treatment of Cells with O. indicum Extracts After CHIKV Infection
In the post-treatment assay, the cells were initially infected with CHIKV at MOI of 0.1. After 1.5 h, the cells were washed with 200 μL of PBS. Following this, different concentration of extracts were added to the cells and incubated for 48 h. The changes in the cells morphology and the viral titre were determined.
Determination of Viral Titre
Viral titres were determined using plaque assay. Vero cells were seeded at density of 3 x 105 per well. The ten-fold dilutions were prepared by adding 100 μL of virus stock in 450 μL of DMEM with 1% FBS. Hundred microliter of each virus dilution were inoculated into each well and incubated for 3 h. Subsequently, 0.5 mL overlay medium containing 3% FBS and 1% carboxymethyl cellulose was added and the plate was incubated for another 3 days. In the present study, overlay medium was used to limit viral spread and allow the formation of discrete plaque formation. At the end of incubation, methylene blue supplemented with 10% formalin was added and left overnight. On the next day, the plate was washed under running tap water and plaques formation was counted and expressed as plaque forming unit per milliliter (pfu/mL).
Statistical Analysis
Statistical analysis was performed using Statistical Package of Social Sciences (SPSS) software, version 22. The experiments were conducted in triplicates and presented as the mean ± standard error (SE). The differences between the groups were analysed using one-way ANOVA and p value (p < 0.05) was considered statistically significant.
Results and Discussion
The toxicity effect of methanol and aqueous extracts of O. indicum leaves on Vero cells was evidenced by the alteration of morphology in the form of cell rounding and nucleus changes compared to normal healthy cells. Cytotoxicity results for both methanol and aqueous extracts are presented in Fig. 1. The cell viability was inversely related with the extract concentration. The viability of methanol-treated Vero cells steadily increased from concentration 5000 μg/mL and below, and at 625 μg/mL, the viability exceeded 90%. The cell viability after aqueous extract exposure steadily increased from 29 to 102% at concentrations of 10 000 μg/mL down to 39 μg/mL, respectively. Similar to methanol treatment, Vero cells also reached > 90% cell viability when treated with aqueous O. indicum extract from concentration of 625 μg/mL downwards. Plant extracts such as, Acmella ciliate had an CC50 of 206 µg/mL [10]. It was considered to be toxic to the cells, as less amount of extract needed to achieve high cells viability. In contrast, the CC50 value for methanol and aqueous extracts of O. indicum were 1875 and 7700 µg/mL, respectively. This indicated that half of the cell viability could be obtained even at higher concentration of extracts, suggesting that O. indicum has lower toxicity towards cells than Acmella ciliate.
In the present study, the MOI which was sufficient to infect all cells in the culture while giving a good replication kinetics and titre were determined. The morphological analysis of infected Vero cells was performed from 12 to 72 h. The cells were infected with various MOIs; 0.05, 0.1, 0.5 and 1.0. CPE formation in Vero cells could be observed when the cells became rounded, shrink, detached from the flask and ultimately disrupted. Results obtained from this study showed that different virus concentration induced different degrees of CPE over time (Table 1). The differences in viral titre and viral replication peak following infection with different MOI are shown in Fig. 2. Infection with MOI of 0.05 resulted in the first CHIKV-infected Vero cells to reach its peak after 12 h of infection by log 6.7 pfu/mL, followed by subsequent rapid cell lost. In contrast, CHIKV replication at MOI of 0.1 gradually increased from 12 to 36 h and peaked to a mean titre of log 6.3 pfu/mL at 48 h post-infection.
CHIKV at MOI of 0.5 was the second to attain peak of replication compare to other MOIs, after 24 h post-infection. Both CHIKV at MOIs of 0.5 and 0.1 reached their peaks at an equal titre of log 6.3 pfu/mL, albeit at different hours post-infection. Plateau phase could be observed until 72 h post-infection for both MOIs. Upon infection with MOI of 1.0, virus titre progressively increased from 12 to 24 h. Then, it replicated to a maximum titre of log 6.0 pfu/mL at 36 h infection, the lowest titre at peak of replication compared to MOIs. At plateau phase, CHIKV at MOI 1 also had the lowest viral titre in comparison to others. CPE started to form after 24 h of infection when high MOIs were used, MOI of 0.5 and 1.0 and displayed 100% CPE as early as second day post-infection. Meanwhile, low MOIs; MOI of 0.1 and 0.05 exhibited 100% CPE after 60 h post-infection. Higher number of MOI increased the exposure of infectious virus particle per exposed cells, suggesting a greater visible effect on Vero cells and could further decrease the time required for cell destruction. We postulated that the degree of CPE over time was prominent, with progressively higher inocula and this evidence was supported by Sourisseau et al. [11]. Anti-viral assay for CHIKV is usually done within 48 h [12, 13]. Therefore, based on the viral replication growth, we chose the infectious dose of MOI of 0.1 to be used on the next subsequent assays as this MOI attained its highest peak titre at 48 h.
Synthetic anti-Chikungunya drugs including chloroquine, ribavirin and arbidol are shown to be potent in cultured cells and animal models [14]. However, these drugs exhibited limited success in human clinical trials. Hence, the search for anti-CHIKV compounds with low toxicity and high inhibitory rate is of utmost importance. In this regards, O. indicum has been tested for its broad pharmacological effects and known to exhibit numerous medicinal properties ranging from being antifungal [15], anti-bacterial [16] and anti-inflammatory [17]. This suggests that O. indicum might be a potential candidate to combat viral infection as well, including Chikungunya. In this study, the concentration of O. indicum extracts used were; 38, 75, 150, 300 and 600 μg/mL for methanol and aqueous extracts, as obtained from the cytotoxicity study. These concentration were tested against CHIKV at MOI of 0.1. The efficacy of anti-CHIKV activities of O. indicum against CHIKV was then evaluated via inhibition of CPE and reduction of virus titre in Vero cells.
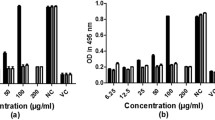
A modified virucidal treatment was performed to identify whether or not, the extracts have the capability to destroy or inactivate the virus directly. However, there was a lack of direct inhibitory effect of methanol extract against CHIKV (p > 0.05). Plaque assay determination of infectious titre showed no inhibition of virus at lower extract concentration. Although no noticeable suppression of CHIKV at lower dose of extract, a slight virus inhibition could be observed at higher concentration of methanol extract. The viral titre was reduced 4.7 and 5.78% upon treatment with 300 and 600 μg/mL of methanol extract, respectively (Fig. 3). In contrast, a reduction of CHIKV titre was observed following exposure with O. indicum aqueous extracts. At lower concentrations, the aqueous extract reduced viral production from log 4.92 pfu/mL to log 4.58 pfu/mL and log 4.52 pfu/mL when CHIKV were exposed to 38 and 75 μg/mL aqueous extract, respectively (Fig. 3). Additionally, viral titres reduced to log 4.58 and log 4.60 upon exposure with 300 and 600 μg/mL of O. indicum aqueous extract, respectively. Interestingly, the highest inhibitory effect, 10% reduction of viral titre, was demonstrated upon exposure with 150 μg/mL aqueous extract (p < 0.05).
Between the two extracts, the aqueous extract exerted a comparatively higher anti-CHIKV effect than the methanol in this study. Still, a reduction of viral titre could be observed in both extracts (Fig. 3). Previously, other compound such as suramin, hesperetin and naringenin are known to inhibit CHIKV replication [18, 19]. However, these compounds did not have direct virucidal effect on CHIKV. The degree of inhibition of these extracts may suggest the presence of bioactive compounds. The presence of these substances in higher concentration could improve the ability of the extracts to further inactivate the virus. Thus, a possible explanation for the low inhibitory activity in this study could be related to insufficient quantity of true anti-viral compounds in the extracts. Further isolation of phytoconstituents of this plant may provide further insight into potential virucidal effect of O. indicum on CHIKV.
To get a clearer picture of the prophylactic effect of O. indicum against CHIKV, the cells were pre-incubated with O. indicum methanol and aqueous extracts prior to viral infection. In the first 24 h, while all pre-treated cells showed no CPE, the virus control displayed 50% CPE. However, after 48 h of infection, both positive control cells and pre-treated cells similarly showed 75% CPE (Table 2). Cytopathic evaluation as demonstrated by the cell morphological changes revealed the ability of pre-treated Vero cells to defer the CHIKV infection only in the first 24 h as after that, at 48 h incubation, the cells showed 75% of CPE which was similar with the positive control cells. These results were in accordance with another study that used Sinbid virus (SINV), in which the CPE formation of infected Vero cells might not essentially affect the virus titre [20].
The alteration in the cells was further confirmed using plaque assay. The titre for positive control was log 5.80 pfu/mL. Nonetheless, the pre-exposure of methanol extract did not inhibit CHIKV replication in Vero cells (p > 0.05) (Fig. 4). On the other hand, there was a detectable CHIKV reduction in the Vero cells pre-treated with aqueous extracts in concentration independent manner. Oroxylum indicum aqueous extract significantly inhibited the replication of CHIKV when the virus was treated with concentration of 150, 300 and 600 μg/mL (p < 0.05). Pre-incubation of Vero cells with these extract concentrations reduced the virus titre from log 7.08 pfu/mL to log 6.80 pfu/mL, log 6.85 pfu/mL and log 6.80 pfu/mL, respectively. However, at lower concentration of the methanol extract, 38 and 75 μg/mL, no reduction of CHIKV titre was observed.
CHIKV titre in infected Vero cells a pre-treated with methanol and aqueous extracts and b post-incubated infected cells with methanol and aqueous extracts. Values were expressed as the mean ± SE for triplicate assays. *There was a statistical difference between control (0 μg/mL) and concentration of 150, 300 and 600 μg/mL (p < 0.05) for pre-treatment assay
The plaque assay results showed that O. indicum aqueous extract had an effect on the viral entry probably due to the presence of various flavonoids that conferred protection to the cells (Fig. 4). The leaf extract of this plant contained several numbers of flavonoid compounds including baicalein and chrysin [21]. Baicalein has been reported to display anti-viral activity against other virus for example, Japanese encephalitis virus [22] and has been suggested to exhibit an inhibitory effect in the early event of CHIKV cycle [23]. Thus, the anti-viral activity of O. indicum could be in part due to the presence of baicalein compound in the extracts. This finding revealed a promising potential of the extract as a prophylactic agent against CHIKV infection by hindering the viral entry into the host cells.
Verification of O. indicum anti-viral action was done by conducting a post-treatment analysis. In this part of study, the CPE development was observed in 24 and 48 h of infection. During the first 24 h, CHIKV-infected Vero cells showed 10% of CPE following exposure with the lowest methanol extract concentration, while Vero cells post-treated with other concentrations showed no CPE development. As for aqueous extract, CPE manifestation ranging from 10% until 25% was be observed in Vero cells post-treated with 38–300 µg/mL extract. However, post-exposure of Vero cells with the highest aqueous concentration resulted in no CPE formation. In contrast, positive controls exhibited greater CPE manifestation (50%). As in pre-treatment assay, similar manifestation of CPE could be observed after 48 h with 75% CPE was observed in all post-treated and positive control cells.
Similar to pre-treatment assay, the CPE manifestation in the post-incubated Vero cells was minimised early in infection and could possibly reduce the viral replication. The CHIKV titre at 48 h post infection on the other hand, showed that both extracts failed in lowering the CHIKV titre. Thus, the post-treatment assay is unlikely to affect the infectious viral synthesis in the cells, suggesting that it possibly had no effect on the late replication stage of CHIKV replication. Similar finding has been reported by Wintachai et al. [23] where no anti-CHIKV activity was observed in cells post-treated with flavaglines and sulfonly amidine compounds.
The plaque assay further supported that the post-treatment of CHIKV infected Vero cells with both extracts exhibited poor inhibitory effect. Post-incubation of methanol extract exerted a certain degree of inhibition at concentration 150 μg/mL by reducing the CHIKV titre from log 6.30 pfu/mL to log 6.07 pfu/mL. Similarly, the aqueous extract inhibited the virus by decreasing the titre from log 6.30 pfu/mL to log 5.88 pfu/mL (150 μg/mL) and log 5.80 pfu/mL (600 μg/mL). However, there were no significant changes of CHIKV titre, suggesting the incapability of these extracts to minimise or inhibit CHIKV multiplication (p > 0.05).
Conclusion
Considering the outcomes obtained from this study, our results provide fresh insight into the treatment of virus using plant remedy. It can be concluded that O. indicum extracts possess some anti-viral effect against CHIKV. The anti-Chikungunya activity was between low to moderate effect, thus suggesting it as a potential candidate for an effective therapy of CHIKV infection. From this study, it could be recommended that perhaps a constant consumption of this plant could act as an aid in the prevention of CHIKV infection.
References
Pineda C, Caballero-uribe CV (2016) Chikungunya in the region of the Americas. A challenge for rheumatologists and health care systems. Clin Rheumatol 35:2381–2385. https://doi.org/10.1007/s10067-016-3390-y
Van Bortel W, Dorleans F, Rosine J, Blateau A, Rousset D, Matheus S, Leparc-Goffart I, Flusin O, Prat C, Cesaire R, Najioullah F, Ardillon V, Balleydier E, Carvalho L, LemaItre A, Noel H, Servas V, Six C, Zurbaran M, Leon L, Guinard A, van den Kerkhof J, Henry M, Fanoy E, Braks M, Reimerink J, Swaan C, Georges R, Brooks L, Freedman J, Sudre B, Zeller H (2014) Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Eurosurveillence. https://doi.org/10.2807/1560-7917.ES2014.19.13.20759
Delatte H, Dehecq JS, Thiria J, Domerg C, Paupy C, Fontenille D (2008) Geographic distribution and developmental sites of Aedes albopictus (Diptera: Culicidae) during a Chikungunya epidemic event. Vector-Borne Zoonotic Dis 8:25–34. https://doi.org/10.1089/vbz.2007.0649
Agarwal A, Joshi G, Nagar DP, Sharma AK, Sukumaran D, Pant SC, Parida MM, Dash PK (2016) Mosquito saliva induced cutaneous events augment Chikungunya virus replication and disease progression. Infect Genet Evol 40:126–135. https://doi.org/10.1016/j.meegid.2016.02.033
Singh V, Chaudhary A (2011) A review on the taxonomy, ethnobotany, chemistry and pharmacology of Oroxylum indicum Vent. Indian J Pharm Sci 73:483–490. https://doi.org/10.4103/0250-474X.98981
Joshi N, Shukla A, Nailwal TK (2014) Taxonomic and phytomedicinal properties of Oroxylum indicum (L.) Vent : a wonderful gift of nature. J Med Plants Res 8:1148–1155. https://doi.org/10.5897/JMPR2013.5178
Tenpe CR, Upaganlawar A, Burle S, Yeole YG (2009) In vitro anti-oxidant and preliminary hepatoprotective activity of Oroxylum indicum Vent leaf extracts. Pharmacologyonline 1:35–43
Mishra S, Sinhamahapatra P, Nayak A, Das R, Sannigrahi S (2010) In vitro anti-oxidant potential of different parts of Oroxylum indicum: a comparative study. Indian J Pharm Sci 72:267–269. https://doi.org/10.4103/0250-474X.65013
Tang LI, Ling AP, Koh RY, Chye SM, Voon KG (2012) Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement Altern Med 12:1–10. https://doi.org/10.1186/1472-6882-12-3
Chan SM, Khoo KS, Sit NW (2015) Interactions between plant extracts and cell viability indicators during cytotoxicity testing: implications for ethnopharmacological studies. Trop J Pharm Res 14:1991–1998
Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, Sol-Foulon N, Le Roux K, Prevost MC, Fsihi H, Frenkiel MP, Blanchet F, Afonso PV, Ceccaldi P, Ozden S, Gessain A, Schuffenecker I, Verhasselt B, Zamborlini A, Saib A, Rey FA, Arenzana-Seisdedos F, Desprès P, Michault A, Albert ML, Schwartz O (2007) Characterization of reemerging Chikungunya virus. PLoS Pathog 3:0804–0817. https://doi.org/10.1371/journal.ppat.0030089
Abdelnabi R, Neyts J, Delang L (2015) Towards anti-virals against Chikungunya virus. Antiviral Res 121:59–68
Lani R, Hassandarvish P, Chiam CW, Moghaddam E, Chu JJH, Rausalu K, Merits A, Higgs S, Vanlandingham D, Bakar SA, Zandi K (2015) Anti-viral activity of silymarin against Chikungunya virus. Sci Rep. https://doi.org/10.1038/srep11421.14
Cruz DJM, Bonotto RM, Gomes RGB, da Silva CT, Taniguchi JB, No JH, Lombardot B, Schwartz O, Hansen MAE, Freitas-Junior LH (2013) Identification of novel compounds inhibiting Chikungunya virus-induced cell death by high throughput screening of a kinase inhibitor library. PLoS Negl Trop Dis 7:e2471. https://doi.org/10.1371/journal.pntd.0002471
Islam MK, Eti IZ, Chowdhury JA (2010) Phytochemical and antimicrobial analysis on the extract of Oroxylum indicum Linn. Stem-Bark. Iran J Pharmacol Ther 9:25–28
Sithisarn P, Nantateerapong P, Rojsanga P, Sithisarn P (2016) Screening for antibacterial and antioxidant activities and phytochemical analysis of Oroxylum indicum fruit extracts. Molecules 21:446–453. https://doi.org/10.3390/molecules21040446
Laloo D, Gogoi B, Lyngdoh W, Zaman K, Sharma HK (2016) Antioxidant, analgesic and anti-inflammatory activities of bark of Oroxylum indicum Vent: an endemic medicinal plant of Northeast India. Asian J Chem 28:2272–2276. https://doi.org/10.14233/ajchem.2016.19968
Albulescu IC, van Hoolwerff M, Wolters LA, Bottaro E, Nastruzzi C, Yang SC, Tsay S-C, Hwu JR, Snijder EJ, van Hemert MJ (2015) Suramin inhibits Chikungunya virus replication through multiple mechanisms. Antiviral Res. https://doi.org/10.1016/j.antiviral.2015.06.013
Ahmadi A, Hassandarvish P, Lani R, Yadollahi P, Jokar A, Bakar SA, Zandi K (2016) Inhibition of Chikungunya virus replication by hesperetin and naringenin. RSC Adv 6:69421–69430. https://doi.org/10.1039/C6RA16640G
Li YG, Siripanyaphinyo U, Tumkosit U, Noranate N, Tao R, Kurosu T, Ikuta T, Takeda N, Anantapreecha S (2012) Chikungunya virus induces a more moderate cytopathic effect in mosquito cells than in mammalian cells. Intervirology 56:6–12. https://doi.org/10.1159/000339985
Yuan Y, Hou W, Tang M, Luo H, Chen LJ, Guan YH, Sutherland IA (2008) Separation of flavonoids from the leaves of Oroxylum indicum by HSCCC. Chromatographia 68:885–892. https://doi.org/10.1365/s10337-008-0859-0
Johari J, Kianmehr A, Mustafa MR, Abubakar S, Zandi K (2012) Anti-viral activity of baicalein and quercetin against the Japanese encephalitis virus. Int J Mol Sci 13:16785–16795. https://doi.org/10.3390/ijms131216785
Wintachai P, Thuaud F, Basmadjian C, Roytrakul S, Ubol S, Desaubry L, Smith DR (2015) Assessment of flavaglines as potential Chikungunya virus entry inhibitors. Microbiol Immunol 59:129–141. https://doi.org/10.1111/1348-0421.12230
Acknowledgements
The authors would like to thank Dr. Wang Seok Mui from Institute of Medical Molecular Biotechnology, Faculty of Medicine, Universiti Teknologi Mara, Selangor, Malaysia, for the kind provision of CHIKV. This study was supported by Fundamental Research Grant Scheme (203.PPSK.6171191).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohamat, S.A., Shueb, R.H. & Che Mat, N.F. Anti-viral Activities of Oroxylum indicum Extracts on Chikungunya Virus Infection. Indian J Microbiol 58, 68–75 (2018). https://doi.org/10.1007/s12088-017-0695-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-017-0695-8