Abstract
Clinical data regarding the prediction of active tuberculosis (TB) development in close TB contacts are scarce. To address this problem, we performed a 2-year follow-up study of Mycobacterium tuberculosis (M.tb) antigen-driven IFN-gamma responses and serum levels of soluble macrophage CD14 receptor in individuals with recent or prolonged M.tb exposure. Between June 2011 and June 2013, we studied 60 healthy Polish adults with recent household or long-term work TB contact and individuals without known M.tb exposure. All of them underwent baseline and repeated testing with IGRA (IFN-gamma release assay) and serum sCD14 ELISA quantification. Frequencies of IGRA results differed at the baseline and follow-up testing. IGRA reversions were noticed in almost one-third of Work TB Contacts and no participants from the Household TB Contact group. IGRA conversions were found in 40 % of Household TB Contacts. No correlation between the IGRA result and the sCD14 level was observed. IFN-γ variability has important implications for clinical practice and requires caution in interpreting the results to distinguish new infections from nonspecific inter-individual variations in cytokine responses. The impairment of IFN-γ response in some individuals with prolonged M.tb exposure representing a resistant immune status does not allow considering IGRA results as reliable and credible. Monitoring the serum sCD14 level can reduce the likelihood of a false prediction of active TB development in close TB contacts showing an M.tb-specific increase in the IFN-gamma production in repeated IGRA testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The infections caused by Mycobacterium tuberculosis (M.tb) are an emerging global problem. Every year, over 8 million people develop TB and nearly 1.3 million die from this disease [1]. The World Health Organization (WHO) predicts that by 2020 one billion people will have become newly infected with M.tb, more than 150 million people will have developed active TB, and 36 million will have died as a result of this disease. The emergence of multi-drug resistance TB remains a major concern of TB programmes worldwide, which has led to an increased need to understand the mechanisms of drug resistance in M.tb and develop new therapeutic strategies [2, 3]. An estimated one-third of the world’s population currently suffers from latent TB infection (LTBI) and can be considered a primary source of TB. LTBI is defined by the evidence of immunological sensitization by M.tb-specific antigens in the absence of clinical symptoms of the disease [4]. It is estimated that in approximately 5 to 10 % of latently M.tb-infected immunocompetent people, the infection will reactivate within their lifetime. The risk of reactivation is significantly increased by immunosuppressive triggers, including HIV infection, anti-tumour necrosis factor alpha (TNF-α), steroid therapies, ageing, malnutrition, renal failure, malignancy or diabetes [4]. However, the identification of latently-infected individuals with the highest risk of progression to active disease is still problematic. The standard technique for diagnosing LTBI is the tuberculin skin test (TST), which measures delayed-type hypersensitivity to a mycobacterial purified protein derivative (PPD), which is present in M.tb, BCG vaccine strains and many non-tuberculous mycobacteria [5]. Therefore, TST positivity can occur as a result of prior BCG immunization or infection with environmental mycobacteria. More specific detection of LTBI has become possible with the development of the in vitro T cell-based interferon-gamma (IFN-γ) release assays (IGRAs) based on the principle that lymphocytes of individuals infected with M.tb produce IFN-γ in response to pathogen-specific antigens, early secretory antigenic target-6 (ESAT-6), culture filtrate protein 10 (CFP-10), and/or TB7.7 [4–7]. These antigens are absent from the M. bovis BCG strains and from the majority of nontuberculous mycobacteria with the exception of M. kansasii, M. szulgai, and M. marinum [8].
The key question is whether IGRAs can distinguish individuals with latent M.tb infection who will develop active TB from those who will not. So far, some prospective studies have evaluated the progression of TB among contacts of infectious pulmonary patients according to IGRA results [9–12]. The major practical problem of this line of research is not the identification of positive results of the test but setting within the positive responses an appropriate cut-off value that might indicate the subsequent development of active TB. To address this problem, we conducted a prospective study in a group of 60 healthy Polish adults with no history of TB and anti-tuberculous treatment, including individuals with recent domestic (Household TB Contacts) or long lasting occupational (Work TB Contacts) TB contact and individuals without known recent M.tb exposure (Community Controls). In parallel with the IFN-γ level analysis, we determined the concentration of the soluble form of the macrophage CD14 receptor (sCD14) in the sera. Our previous study showed that a significant increase in the serum sCD14 level characterizes patients with active TB, suggesting that increased sCD14 can be a potential biomarker for TB development [13]. From this perspective, it seemed interesting to compare the sCD14 concentration in the sera of volunteers who had undergone conversion or reversion of their IGRA results.
Materials and Methods
Study Population
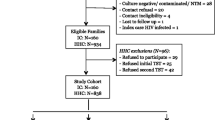
In May 2011, we identified a group of 60 volunteers consisting of 29 individuals recruited from the medical staff (doctors and nurses directly caring for patients with pulmonary TB) of the Specialized Hospital of Tuberculosis and Lung Diseases in Lodz, Poland (Work TB Contacts), 15 subjects who were family members of patients with newly diagnosed active pulmonary TB (Household TB Contacts), and 16 volunteers without any TB history and any known exposure to infectious TB (Community Controls), recruited from the employees of the Institute for Microbiology and Immunology, University of Lodz, Lodz, Poland. All of the volunteers showed normal chest X-ray results and had no clinical symptoms of TB. All of the studied subjects had been vaccinated with BCG in the past according to the Government’s Health Program recommendations and were HIV-negative. None of the volunteers suffered from TB or had ever received anti-TB therapies. However, in the Work TB Contact group one volunteer suffered from coronary heart disease, four had hypertension, one suffered from glaucoma and one from house dust allergy. Among Household TB Contacts, one participant suffered from hypertension and one person had an allergy to house dust and pet’s dander. These participants took daily medications. None of the studied healthy people reported any health problems or took any medications. The volunteers underwent IGRA testing and serum sCD14 quantification twice (1) between May and June 2011 and (2) between June and July 2013. Identical testing protocols were used for the assays performed in 2011 and 2 years later. Written consent was provided by all individuals before blood donation, and the study protocol was approved by the Bioethics Committee of the Medical University of Lodz, Poland.
Interferon-γ Release Assay (IGRA)
The QuantiFERON-TB Gold In-Tube kit (QFT; Cellestis Ltd, Carnegie, Australia) for the quantitative assessment of IFN-γ was performed and interpreted according to the manufacturer’s instructions. In our study, the incubation of blood in the presence of mycobacterial antigens or mitogen was initiated within 3 h after blood collection. The assay result was considered positive if the IFN-γ concentration of TB-Ag—Nil was ≥0.35 IU/ml. Of note, 1 IU/ml IFN-γ is equivalent to 40 pg/ml [14]. IGRA conversion was taken into consideration when the baseline IFN-γ level was <0.35 IU/ml (14 pg/ml) and the follow-up IFN-γ concentration was ≥14 pg/ml; IGRA reversion was defined when the baseline IFN-γ was ≥14 pg/ml and the follow-up IFN-γ was <14 pg/ml.
Serum sCD14 Concentration
The levels of sCD14 in the sera were measured using an ELISA-based capture assay (Human sCD14 Quantikine Kit, R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The sensitivity of the assay was 125 pg sCD14/ml.
Statistical Analysis
Data were entered into a Microsoft Excel spreadsheet and then copied into the Statistica 5.0 software program (Statsoft) for statistical analysis. Comparisons of the proportions were tested for the significance of difference using the χ2 test or the Fisher exact test. The Kruskal–Wallis Anova test was used to identify statistically significant differences in the levels of IFN-γ and sCD14. Differences were considered significant when p < 0.05.
Results
Baseline Characteristics of Participants
A total of 60 Polish participants consented to take part in the study (Table 1). The mean age of the Work TB Contacts, Household TB Contacts and Community Controls was comparable at 43.7 (range 31–56), 40.1 (range 18–60) and 47.3 (range 27–61) years, respectively. The majority of the volunteers were women, with 96.5, 53.3 and 81.2 % women in the Work TB Contacts, Household TB Contacts and Community Controls, respectively. Work TB Contacts had a documented TB exposure during the cumulative working time in the Specialized Hospital of Tuberculosis and Lung Diseases. Twenty (68 %) of them had worked in a TB healthcare sector as medical staff for more than 10 years (Table 1).
IGRA Results at the Baseline and Follow-up Testing
Figure 1 shows the frequency of positive and negative IGRA results in the studied Contact and Control groups at the baseline and follow-up testing. No indeterminate IGRA results were noted during the study. The prevalence of positive IGRA results observed at baseline in the Work TB Contact and Household TB Contact groups was 55 and 33 %, respectively. No volunteers from Community Controls were IGRA-positive at the beginning of the study. At the follow-up testing of individuals with negative IGRA results at baseline, 1 out of 13 (8 %) Work TB Contacts, 4 out of 10 (40 %) Household TB Contacts, and 1 out of 16 (7 %) Community Controls became IGRA-positive. The reversions of IGRA results were observed in 5 out of 16 (31 %) individuals from the Work TB Contact group only, and the reversion rate was the highest (67 %) among individuals with low baseline IFN-γ levels ranging from 14 to 40 pg/ml. In total, 2 years after the start of the study, the prevalence of IGRA-positives decreased in the Work TB Contact group (41 %) and increased in the Household TB Contact group (60 %) (Fig. 1). No Contacts or Controls developed active TB during the study period. The daily medications by volunteers with hypertension, coronary heart disease, glaucoma or allergy to house dust remained unconnected with the reversion of IGRA results. None of 3 IGRA-positive volunteers suffering from these diseases became IGRA-negative during the study period.
IFN-γ Response at the Baseline and Follow-up Testing
The IFN-γ values obtained for M.tb antigen-stimulated blood cultures (QFT-ELISA) were normalized against the background by subtracting the value obtained for the respective negative controls. The results are shown as an absolute mean value ±SD of the cytokine concentration (pg/ml) in each group (Fig. 2). At baseline, a significantly lower production of IFN-γ was observed in the Community Controls (0.28 ± 0.43 pg/ml) than in the Work TB Contacts (46.6 ± 79.3 pg/ml), p = 0.000002, and the Household TB Contacts (78.1 ± 147.6 pg/ml), p = 0.04. At the follow-up testing, M.tb-stimulated leukocytes from the Household TB Contacts and the Work TB Contacts produced IFN-γ close to the baseline results, at, 46.5 ± 86.1 and 94.3 ± 134.9 pg/ml, respectively. The highest mean IFN-γ concentration was observed among the Work TB Contacts with prolonged M.tb exposure (64.3 ± 94.7 and 72.8 ± 104.9 pg/ml at baseline and follow-up testing, respectively), while the lowest was observed among those who had worked in the TB ward for less than 5 years (11.3 ± 16.1 pg/ml at baseline and 19.2 ± 28.4 pg/ml at follow-up), however, the observed differences were not statistically significant.
Serum sCD14 Levels at Baseline and Follow-up Testing
As shown in Fig. 3a, the serum sCD14 contents measured at baseline in the Work TB Contacts, Household TB Contacts and Community Controls were similar (1571 ± 319, 1924 ± 304, and 1599 ± 247 ng/ml, respectively). 2 years after recruitment, the levels of soluble CD14 in the sera were also comparable (1445 ± 193, 1581 ± 256, and 1573 ± ng/ml, respectively) and the values did not differ from those observed at baseline. There were no statistically significant differences in the mean sCD14 concentration in the sera of IGRA-negative and IGRA-positive individuals measured at the baseline and follow-up (Fig. 3b). The individual serum sCD14 levels were not associated either with the conversion or reversion of IGRA testing (Table 2). Moreover, a duration of employment in a TB healthcare sector did not influence the serum sCD14 concentration. The concentrations of sCD14 in the sera from the Work TB Contacts who had worked in the hospital for more than 10 years (1533 ± 315 ng/ml at baseline and 1437 ± 189 ng/ml at follow-up) were comparable to the sCD14 levels in the sera from those, who had cared for TB patients for less than 5 years (1816 ± 351 ng/ml at baseline and 1538 ± 340 ng/ml at follow-up).
Discussion
Persons who have had a close contact with infectious pulmonary TB represent a high-risk group, for which the accurate detection of LTBI is believed to be of great importance. Currently, the diagnosis of LTBI relies on specific M.tb antigen-driven IFN-γ responses measured in IGRA assays and flow cytometry assays of M.tb antigen driven effector T cell responses [5, 15]. In our longitudinal study, we assessed IFN-γ responses to specific M.tb antigens (ESAT-6, CFP-10, TB7.7) using QFT, in 60 healthy Polish volunteers with recent domestic or long lasting occupational TB contact and those not exposed to infectious TB. The frequency of IGRA-positive results at the baseline testing was the highest among the Work TB Contacts (55 %). Menzies et al. [16] reported that the median prevalence of LTBI in this occupational group was 63 % (range 33–79 %) in low- and middle-income countries, and 24 % (range 4–46 %) in high-income countries. In our study, approximately 80 % of the studied Work TB Contacts had concordant IGRA results at baseline and follow-up studies. Unexpectedly, at follow-up testing, almost one-third (5 out of 16) of the initially IGRA-positive individuals in this group were found to be IGRA-negative. Similarly, IGRA reversions of unknown origin were described in a study of Pai et al., performed on healthcare workers in India [17]. The rate of IGRA reversions ranged from 7 % in the IGRA-positive/TST-positive baseline individuals to 70 % in the IGRA-positive/TST-negative individuals. In addition, Park et al. [18] showed monthly fluctuations in IFN-γ levels in the group of health-care workers studied for 1 year and observed discordant results in serial testing in 25 out of 48 (52 %) participants. In other serial IGRA studies on health-care workers, the IGRA reversion rate ranged from 25 to 41 % [19, 20]. In our study, the reversions were noticed in 5 out of 16 initially IGRA-positive Work TB Contacts, and in contrast, in no participants from the Household TB Contact group. The biological explanation for the reversions remains unclear. Some authors have suggested that IGRA reversions might be a result of the healing of TB infection, the transition of mycobacteria into a dormant state or the periodic secretion of antigens from M.tb [18, 20]. In our opinion, IGRA reversions could be partly due to the differences in the immune status of studied individuals, responsible for a diverse course of antimycobacterial response. It is accepted that host genetic background plays a role in the susceptibility to TB, restricting the infection or leading to active TB development [21]. The genetically determined mechanisms that underlie the initiation and maintenance of immune responses against M.tb involve many different immune parameters as well as various immune cell types [22, 23]. Genetic differences that affect the functions of these cells can generate the imbalance between M.tb and the host immunity, thus influencing the outcome of the mycobacteria-host interactions. IFN-γ producing cells, such as CD4+ and CD8+ T cells, natural killer (NK) cells, γ/δ T cells, CD1-restricted T cells, are key elements of the protective immune response against mycobacteria due to the fact that they might increase the killing strength of macrophages [24]. Dominant M.tb-driven IFN-γ producing subsets can differ among individuals. In our study (not published), in M.tb-stimulated dendritic cell—lymphoid cell co-cultures conducted for some BCG-vaccinated healthy individuals, intracellular IFN-γ was detected mainly in CD8+ T or NK cells, while in the other cocultures IFN-γ was produced by both CD4+ and CD8+ T cells. Additionally, macrophages present in whole blood IGRA cultures were found to regulate IFN-γ production by cytokines, such as IL-12, IL-18 and IL-27 [25]. The results of our study showed that initially enhanced IFN-γ production observed in some Work TB Contacts weakened over time, however it seemed to be effective enough to contain the development of active disease. For this reason, IGRA results might sometimes be unreliable in the long-term M.tb exposed Work TB Contacts, especially in those who represent a resistant immune status.
The conversion of IGRA is considered to be a confirmation of M.tb infection. In our study, IGRA conversion was significantly more frequent in the Household TB Contacts (40 %) than in the Work TB Contacts (8 %) and the Community Controls (6 %). Various rates of IGRA conversion have been documented among contacts of active TB cases depending on the test and definitions used [17, 20]. It is suggested that IGRA may have suboptimal sensitivity to detect recent M.tb infections, especially in TB high-risk groups such as the homeless, malnourished, drug addicts, alcohol abusers. Household TB Contacts that consented to take part in our study came from normal Polish families with an average standard of living. The higher percentage of converters in the Household TB Contact group might have been a consequence of too short an interval between initial M.tb exposure and IGRA testing. The limited sensitivity of IGRA tests, preventing detection of weak mycobacteria-driven immune responses, justifies the search for new antigens with potent immunostimulatory activity and novel diagnostic methods for rapid identification of M.tb infections, in particular the most dangerous ones such as tuberculous meningitis [26–28]. A recent study by Fong et al. [29] showed that IGRA conversions in serial testing remained a challenging task for clinicians and that using single cut-off point criteria for IGRA might lead to a significant number of false-positive results and overdiagnosis of LTBI. In the opinion of other researchers, an M.tb-specific increase in the IFN-γ production might be interpreted as a risk factor for developing active TB among IGRA-positive individuals [30]. Individuals with a strong IFN-γ response to the ESAT-6 compared to those with low IFN-γ response had a tenfold increased risk of subsequently developing clinical TB in the 1–2-year period after the exposure [11]. This observation was consistent with the fact that successful antituberculosis therapy was found to decrease the level of IFN-γ in response to specific M.tb antigens, ESAT-6 and CFP-10 [31–33]. Adetifa et al. [34] reported a rising qualitative and quantitative ELISPOT counts of IFN-γ producing T lymphocytes in response to ESAT-6/CFP-10 peptides in a close TB contact in Gambia, who developed active disease over a 5-month period. The quantitative PPD-based ELISPOT assay was also found to be useful for the assessment of the infectious load of M.tb as a result of recent TB exposure [35, 36]. Hill et al. [36] showed an association between recent M. tuberculosis exposure and an elevated immune response to PPD as measured by the ELISPOT count or skin test reaction.
Trying to explain whether the M.tb-specific increase in IFN-γ production in IGRA-positive individuals can be interpreted as a risk factor for the development of active TB, we determined serum levels of a soluble form of the macrophage CD14 receptor (sCD14). The most crucial event in innate immunity to M.tb is the activation of macrophages. It is triggered by the specific binding between bacterial components and pattern recognition receptors, including CD14 molecules. CD14 is present in two forms: membrane-bound CD14 (mCD14), expressed on the surface of monocytes/macrophages, and soluble CD14 (sCD14), found in the circulation. CD14 is involved in the interactions with many M.tb ligands and initiates a variety of effector functions such as production of proinflammatory cytokines and upregulation of cell adhesion molecules [13, 37]. Much evidence has suggested that CD14 contributes to inflammatory processes in the lung. CD14 knock-out mice were protected from mortality and displayed reduced lung inflammation upon infection with M.tb [37]. In patients with the most aggressive forms of TB high levels of sCD14 decreased to normal levels after anti-tuberculosis treatment [38].
To date, the effect of LTBI on the circulating sCD14 levels has not been studied extensively. Our previous study showed a simultaneous increase in the monocyte expression of the mCD14 receptor and LFA-1 integrin in active TB, which might be considered a prodrome of the loss of immune control due to M.tb bacilli in IGRA-positive individuals [13]. At the same time, patients with active pulmonary TB showed a significant increase in the level of serum sCD14 (2199 ± 373 ng/ml) compared to healthy subjects (1644 ± 293 ng/ml) [13]. An increased level of serum sCD14 in patients with TB was confirmed by Pacheco et al., who showed that those with miliary TB had the highest sCD14 concentrations [39]. However, our study did not reveal any differences in the serum levels of sCD14 at particular in different stages of active TB. In culture-positive patients with mild (lesions within one-third of the unilateral lung field), moderate (lesions within the unilateral lung field) or advanced (lesions beyond the unilateral lung field) TB, the serum sCD14 levels were comparable, at 2045 ± 472, 2294 ± 247 or 2223 ± 265 ng/ml, respectively. On the contrary, comparing the concentrations of IFN-γ in different TB groups, the M.tb culture-positive patients with advanced forms of TB were characterized by a lower IFN-γ level (1.00 ± 1.06 IU/ml) than those with mild (4.59 ± 4.77 IU/ml) or moderate (3.45 ± 3.49 IU/ml) TB forms [7]. Elevated levels of sCD14 as such cannot be considered to be specific indicators of active TB, because increased serum sCD14 concentrations were also observed in nonmycobacterial lung infections as well as in many noninfectious diseases [13, 40]. In this way, sCD14 appears as an acute-phase protein. In active TB, the elevated sCD14 levels could have been a consequence of non-resolving inflammation. However, in this study, no increase in the concentrations of soluble CD14 level were observed at baseline and follow-up testing in the sera of individuals who underwent either conversion or reversion of the IGRA result. There was also no association noted between the serum level of sCD14 and the IFN-γ levels obtained by IGRA testing. It suggests some caution in the interpretation of the observed increase in the IFN-γ level measured by IGRA as a factor predisposing to the development of active TB.
In conclusion, our data confirm that the measurement of the specific M.tb antigen-driven IFN-γ response is a relevant method of detecting latent M.tb infections among TB contacts, especially in a country like Poland with high BCG vaccination coverage and intermediate levels of M.tb exposure. However, IFN-γ variability has important implications for clinical practice and requires caution in interpreting the results to distinguish new infections from nonspecific inter-individual variations in cytokine responses. The impairment of IFN-γ response in some individuals with prolonged M.tb exposure representing a resistant immune status does not allow considering IGRA results as reliable and credible. Monitoring the level of serum sCD14 can reduce the likelihood of a false prediction of active disease in close TB contacts showing an M.tb-specific increase in the IFN-γ production in repeated IGRA testing.
References
World Health Organization.org [Internet]. Global tuberculosis report 2014. [updated 2015 16 July; cited 2015 September 24]. http://www.who.int/tb/publications/global_report/en/
Saxena A, Mukherjee U, Kumari R, Singh P, Lal R (2014) Synthetic biology in action: developing a drug against MDR-TB. Indian J Microbiol 54:369–375. doi:10.1007/s12088-014-0498-0
Phong TQ, do Ha TT, Volker U, Hammer E (2015) Using a label free quantitative proteomics approach to identify changes in protein abundance in multidrug-resistant Mycobacterium tuberculosis. Indian J Microbiol 55:219–230. doi:10.1007/s12088-015-0511-2
Gideon HP, Flynn JL (2011) Latent tuberculosis: What the host “sees”? Immunol Res 50:202–212. doi:10.1007/s12026-011-8229-7
Horvat RT (2015) Review of interferon gamma assays used in the diagnosis of tuberculosis. Clin Vaccine Immunol 22:845–849. doi:10.1128/CVI.00199-15
Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, Bossink A, Dheda K, Diel R, Dominguez J, Lipman M, Nemeth J, Ravn P, Winkler S, Hiutric E, Sandgren A, Manissero D (2011) Interferon-release assays for the diagnosis of active tuberculosis: a systemic review and meta-analysis. Eur Respir J 37:100–111. doi:10.1183/09031936.00114810
Wlodarczyk M, Rudnicka W, Janiszewska-Drobinska B, Kielnierowski G, Kowalewicz-Kulbat M, Fol M, Druszczynska M (2014) Interferon-gamma assay in combination with tuberculin skin test are insufficient for the diagnosis of culture-negative pulmonary tuberculosis. PLoS One 15:e107208. doi:10.1371/journal.pone.0107208
Kim MH, Kim YH, Kang SY, Lee WI (2015) The incidence of non-tuberculous Mycobacterium lung disease in patients with suspected pulmonary tuberculosis. Indian J Microbiol 55:464–468. doi:10.1007/s12088-015-0543-7
Bakir M, Millington KA, Soysal A, Deeks JJ, Efee S, Aslan Y, Dosanjh DP, Lalvani A (2008) Prognostic value of a T-cell based, interferon-gamma biomarker in children with tuberculosis contact. Ann Intern Med 2:777–787
Doherty TM, Demissie A, Olobo J, Wolday D, Britton S, Eguale T, Ravn P, Andersen P (2002) Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J Clin Microbiol 40:704–706
Diel R, Loddenkemper R, Nienhaus A (2012) Predictive value of interferon-γ release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest 142:63–75. doi:10.1378/chest.11-3157
Andersen P, Doherty TM, Pai M, Weldingh K (2007) The prognosis of latent tuberculosis: Can disease be predicted? Trends Mol Med 13:175–182
Druszczynska M, Wlodarczyk M, Janiszewska-Drobinska B, Kielnierowski G, Zawadzka J, Kowalewicz-Kulbat M, Fol M, Szpakowski P, Rudnicka K, Chmiela M, Rudnicka W (2013) Monocyte signal transduction receptors in active and latent tuberculosis. Clin Dev Immunol 2013:851452. doi:10.1155/2013/851452
Desem N, Jones SL (1998) Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol 5:531–536
Wyndham-Thomas C, Corbiére V, Dirix V, Smits K, Domont F, Libin M, Loyens M, Locht C, Mascart F (2014) Key role of effector memory CD4+ T lymphocytes in a short-incubation heparin-binding hemagglutinin gamma interferon release assay for the detection of latent tuberculosis. Clin Vaccine Immunol 21:321–328. doi:10.1128/CVI.00651-13
Menzies D, Joshi R, Pai M (2007) Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis 11:593–605
Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Kalentri S, Reingold AL, Colford JM Jr, Riley LW, Menzies D (2006) Serial testing of health care workers for tuberculosis using interferon-γ assay. Am J Respir Crit Care Med 174:349–355
Park JS, Lee JS, Kim MY, Lee CH, Yoon HI, Lee SM, Yoo CG, Kim YW, Han SK, Yim JJ (2012) Monthly follow-ups of interferon-γ release assays among health-care workers in contact with patients with TB. Chest 142:1461–1468
Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M (2012) Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax 67:62–70. doi:10.1136/thx.2010.143180
Hill PC, Brookes RH, Fox A, Jackson-Sillah D, Jeffries DJ, Lugos MD, Donkor SA, Adetifa IM, de Jong BC, Aiken AM, Adegbola RA, McAdam KP (2007) Longitudinal assessment of an ELISPOT test for Mycobacterium tuberculosis infection. PLoS Med 4:e192
Fol M, Druszczynska M, Wlodarczyk M, Ograczyk E, Rudnicka W (2015) Immune response gene polymorphisms in tuberculosis. Acta Biochim Pol 62:633–640. doi:10.18388/abp.2015_1130
Orme IM, Robinson RT, Cooper AM (2015) The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol 16:57–63. doi:10.1038/ni.3048
Maji A, Misra R, Mondal AK, Kumar D, Bajaj D, Singhal A, Arora G, Bhaduri A, Sajid A, Bhatia S, Singh S, Singh H, Rao V, Dash D, Shalini EB, Sarojini MJ, Chaudhary A, Gokhale RS, Singh Y (2015) Expression profiling of lymph nodes in tuberculosis patients reveal inflammatory milieu at site of infection. Sci Rep 15:15214. doi:10.1038/srep15214
Ottenhoff THM (2012) The knowns and unknowns of the immunopathogenesis of tuberculosis. Int J Tuberc Lung Dis 16:1424–1432. doi:10.5588/ijtld.12.0479
Robinson CM, O’Dee D, Hamilton T, Nau GJ (2010) Cytokines involved in interferon-γ production by human macrophages. J Innate Immun 2:56–65. doi:10.1159/000247156
Cai L, Zhao X, Jiang T, Qiu J, Owusu L, Ma Y, Wang B, Xin Y (2014) Prokaryotic expression, identification and bioinformatics analysis of the Mycobacterium tuberculosis Rv3807c gene encoding the putative enzyme committed to decaprenylphosphoryl-D-arabinose synthesis. Indian J Microbiol 54:46–51. doi:10.1007/s12088-013-0418-8
Nagdev KJ, Bhagchandani SP, Bhullar SS, Kapgate RC, Kashyap RS, Chandak NH, Daginawala HF, Purohit HJ, Taori GM (2015) Rapid diagnosis and simultaneous identification of tuberculous and bacterial meningitis by a newly developed duplex polymerase chain reaction. Indian J Microbiol 55:213–218. doi:10.1007/s120088-015-0517-9
Szewczyk R, Kowalski K, Janiszewska-Drobinska B, Druszczynska M (2013) Rapid method for Mycobacterium tuberculosis identification using electrospray ionization tandem mass spectrometry analysis of mycolic acids. Diagn Microbiol Infect Dis 76:298–305. doi:10.1016/j.diagmicrobio.2013.03.025
Fong KS, Tomford JW, Teixeira L, Fraser TG, van Duin D, Yen-Lieberman B, Gordon SM, Miranda C (2012) Challenges of interferon-gamma release assay conversions in serial testing of health-care workers in a TB control program. Chest 142:55–62. doi:10.1378/chest.11-0992
Higuchi K, Harada N, Fukazawa K, Mori T (2008) Relationship between whole-blood interferon-gamma responses and the risk of active tuberculosis. Tuberculosis 88:244–248. doi:10.1016/j.tube.2007.11.009
Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, Hill AV, Lalvani A (2001) Direct ex vivo analysis of antigen-specific IFN-γ-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol 167:5217–5225
Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D (2004) Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis 38:754–756
Lee SH, Lew WJ, Kim HJ, Lee HK, Lee YM, Cho CH, Lee EJ, Lee DY, Ryu SW, Oh SY, Kim SO, Shim TS (2010) Serial interferon-gamma release assays after rifampicin prophylaxis in a tuberculosis outbreak. Respir Med 104:448–453. doi:10.1016/j.rmed.2009.10.006
Adetifa IM, Brookes R, Lugos MD, de Jong BC, Antonio M, Adegbola RA, Hill PC (2007) Rising ELISPOT count prior to the onset of symptoms of full-blown tuberculosis disease. Int J Tuberc Lung Dis 11:350–352
Hill PC, Fox A, Jeffries DJ, Jackson-Sillah D, Lugos MD, Owiafe PK, Donkor SA, Hammond AS, Corrah T, Adegbola RA, McAdam KP, Brookes RH (2005) Quantitative T cell assay reflects infectious load of Mycobacterium tuberculosis in an endemic case contact model. Clin Infect Dis 40:273–278
Hill PC, Jackson-Sillah DJ, Fox A, Brookes RH, de Jong BC, Lugos MD, Adetifa IM, Donkor SA, Aiken AM, Howie SR, Corrah T, McAdam KP, Adegbola RA (2008) Incidence of tuberculosis and the predictive value of ELISPOT and Mantoux tests in Gambian case contacts. PLoS One 3:e1379. doi:10.1371/journal.pone.0001379
Wieland CW, van der Windt GJ, Wiersinga WJ, Florquin S, van der Poll T (2008) CD14 contributes to pulmonary inflammation and mortality during murine tuberculosis. Immunology 125:272–279. doi:10.1111/j.1365-2567.2008.02840.x
Lawn SD, Labeta MO, Arias M, Acheampong JW, Griffin GE (2000) Elevated serum concentrations of soluble CD14 in HIV- and HIV + patients with tuberculosis in Africa: prolonged elevation during anti-tuberculosis treatment. Clin Exp Immunol 120:483–487
Pacheco E, Fonseca C, Montes C, Zabaleta J, Garcia LF, Arias MA (2004) CD14 gene promoter polymorphism in different clinical forms of tuberculosis. FEMS Immunol Med Microbiol 40:207–213
Takeshita S, Nakatani K, Tsujimoto H, Kawamura Y, Kawase H, Sekine I (2000) Increased levels of circulating soluble CD14 in Kawasaki disease. Clin Exp Immunol 119:376–381
Acknowledgments
This work was supported by the grant from the Polish Ministry of Science and Higher Education (N N402 098539) and co-financed the European funds for development of the region of Lodz (No. 5/SMN/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Druszczynska, M., Wlodarczyk, M., Kielnierowski, G. et al. Two-Year Follow-up Study of Mycobacterium tuberculosis Antigen-Driven IFN-γ Responses and Macrophage sCD14 Levels After Tuberculosis Contact. Indian J Microbiol 56, 205–213 (2016). https://doi.org/10.1007/s12088-016-0571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-016-0571-y