Abstract
The importance of the extracellular matrix (ECM) in fibrosis has been recognized for a long time, not only because ECM’s increased stiffness hampers tissue function, but also because the ECM provides the mechanical tension that maintains resident cells’ synthetic phenotype. A study by Parker and colleagues (Journal of Clinical Investigation 124, 1622–1635, 2014) compared the transcriptome of fibroblasts cultured on decellularized ECM from healthy vs idiopathic pulmonary fibrosis (IPF) human lungs, and revealed that the IPF matrix exerts a positive feedback loop that increases the translation of ECM genes that are enriched in the IPF ECM proteome. This study suggests that the ECM composition, in addition to ECM stiffness or the phenotype of its resident cells, might be an important factor in maintaining a fibrotic state. Targeting this feedback loop might be an efficient therapeutic strategy for IPF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
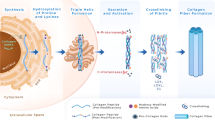
Extracellular matrix (ECM) deposition initially intensifies as a reparative response to insult or injury (such as in the form of a scar after cutaneous wounding). When prolonged or excessive, the phenomenon becomes pathogenic fibrosis. The resulting thickened ECM hampers tissue properties and may lead to organ failure (Wynn 2008; Friedman et al. 2013). Fibrotic diseases are often of unclear etiology, but many share common features such as the presence of myofibroblasts (Hinz et al. 2007; Falke et al. 2015), inflammatory and immune infiltrates (Wick et al. 2013), and accumulation or activation of pro-fibrotic cytokines and other matricellular proteins (Jun and Lau 2011; Luzina et al. 2014). While cell types and soluble factors are keys to the pathophysiology of fibrosis, it is increasingly clear that the ECM itself exerts a critical role in maintaining resident cells’ phenotype. ECM-generated mechanical tension triggers synthesis of ECM proteins in fibroblasts (Mauch et al. 1988; Kessler et al. 2001), and reduced mechanical tension in aged skin decreases levels of ECM-building proteins (Varani et al. 2006; Rittié and Fisher 2015). The mechano-signaling pathways that regulate these phenomena are becoming better elucidated (Duscher et al. 2014), and attempts to block these pathways in fibrosis are thus far promising (Lagares et al. 2012; Zhou et al. 2013). Meanwhile, 2014 revealed another dimension to the importance of the ECM in fibrosis (Parker et al. 2014).
Parker et al. studied idiopathic pulmonary fibrosis (IPF), a disease characterized by progressive scarring of the lungs that leads to death by asphyxia. In IPF, both ECM and resident fibroblasts harbor the common fibrotic features listed above. In their study, Parker et al. compared the transcriptome of control vs IPF fibroblasts cultured on decellularized ECM from healthy vs IPF human lungs. They show that decellularized ECM from IPF lungs triggered ECM synthesis in lung fibroblasts to a greater extent than that of healthy lung ECM. These results were expected due to the greater stiffness of fibrotic matrix compared to healthy ECM (Liu et al. 2010; Liu et al. 2014). However, Parker et al. also show that, when cultured on IPF ECM, fibroblasts increase the translation of a set of RNAs that encode proteins that are enriched in the IPF ECM proteome. Why were IPF-specific ECM proteins more translated than other ECM genes? This intriguing observation was partially explained by demonstrating that miR-29c, which targets are significantly over-represented in the IPF ECM proteome, was reduced when fibroblasts were cultured on IPF vs healthy ECM (miR29c levels are also dramatically reduced in both rapidly- and slowly-progressing IPF in vivo (Oak et al. 2011)). There are likely additional mechanisms responsible for this observation. Nevertheless, these results are important because they suggest that the ECM composition provides a positive feedback for resident cells in instructing them to produce the components needed to sustain a diseased state.
In conclusion, Parker et al. exposed the interesting observation that the composition of an ECM might be an equally important controller of fibrosis as is the ECM stiffness or the phenotype of its resident cells. Additional work to address the details of specific genes or other fibrotic conditions is guaranteed.
References
Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, Hu M, Whitmore AJ, Whittam AJ, Longaker MT, Gurtner GC (2014) Mechanotransduction and fibrosis. J Biomech 47:1997–2005
Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ (2015) Diverse origins of the myofibroblast-implications for kidney fibrosis. Nat Rev Nephrol
Friedman SL, Sheppard D, Duffield JS, Violette S (2013) Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 5:167sr161
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170:1807–1816
Jun JI, Lau LF (2011) Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 10:945–963
Kessler D, Dethlefsen S, Haase I, Plomann M, Hirche F, Krieg T, Eckes B (2001) Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem 276:36575–36585
Lagares D, Busnadiego O, Garcia-Fernandez RA, Kapoor M, Liu S, Carter DE, Abraham D, Shi-Wen X, Carreira P, Fontaine BA, Shea BS, Tager AM, Leask A, Lamas S, Rodriguez-Pascual F (2012) Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum 64:1653–1664
Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ (2014) Mechanosignaling through YAP and TAZ drive fibroblast activation and fibrosis. American journal of physiology. Lung cellular and molecular physiology. ajplung 00300 02014
Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ (2010) Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190:693–706
Luzina IG, Todd NW, Sundararajan S, Atamas SP (2014) The cytokines of pulmonary fibrosis: Much learned, much more to learn. Cytokine
Mauch C, Hatamochi A, Scharffetter K, Krieg T (1988) Regulation of collagen synthesis in fibroblasts within a three-dimensional collagen gel. Exp Cell Res 178:493–503
Oak SR, Murray L, Herath A, Sleeman M, Anderson I, Joshi AD, Coelho AL, Flaherty KR, Toews GB, Knight D, Martinez FJ, Hogaboam CM (2011) A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One 6:e21253
Parker MW, Rossi D, Peterson M, Smith K, Sikstrom K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB (2014) Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Investig 124:1622–1635
Rittié L, Fisher GJ (2015) Natural and sun-induced aging of human skin. Cold spring harbor perspectives in medicine 5: a015370
Varani J, Dame MK, Rittié L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ (2006) Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol 168:1861–1868
Wick G, Grundtman C, Mayerl C, Wimpissinger TF, Feichtinger J, Zelger B, Sgonc R, Wolfram D (2013) The immunology of fibrosis. Annu Rev Immunol 31:107–135
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ (2013) Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Investig 123:1096–1108
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rittié, L. Another dimension to the importance of the extracellular matrix in fibrosis. J. Cell Commun. Signal. 9, 99–100 (2015). https://doi.org/10.1007/s12079-015-0282-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-015-0282-x