Abstract
Background and Aim
Eating is one of our basic needs, and food can be extremely rewarding. Different aspects of food can elicit the brain’s reward system. In the present study we set out to investigate differences in brain activity evoked by pleasant food and non-food odors, with a particular focus on dopaminergic brain regions.
Material and Methods
We measured cerebral activity in 23 participants by means of magnetic resonance imaging, who were stimulated with three food odors (strawberry, orange, and mango) and three non-food odors (lily of the valley, jasmine, and lavender). We acquired functional images using a block stimulation design. Participants also evaluated pleasantness, intensity, and edibility of the odorants.
Results
Food odors elicited larger activations in the left postcentral gyrus, the left superior frontal gyrus, and in the midbrain. However, despite careful piloting, food odors were on average rated as significantly more intense than the flower odors. We therefore restricted the subsequent analysis to odors which were matched with regard to intensity and pleasantness. Thus, comparing strawberry and lavender odor yielded significant activations in the right cingulate, the midbrain, and the insula bilaterally. We further extracted the percentage of signal change from the global mean (beta) for all odorants in all subjects’ ventral tegmental area, which revealed stronger signal change when subjects smelled food odors compared to flower odors.
Discussion
These results indicate that food-related odors may activate dopaminergic pathways in the brain, possibly due to a conditioned association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eating is one of our basic needs, and food can be extremely rewarding. In fact, feeding may release dopamine in brain regions considered to be reward-processing areas (e.g. (Small et al. 2003)). Further, feeding is not necessary for the activation of these areas, as even food display including the smell, the taste, and the sight of food, but without ingestion can lead to dopamine release (Volkow et al. 2002). In a meta-analysis comparing images of food vs neutral cues and images of smoking vs neutral cues, it has been shown that both stimuli activated a similar reward-cuing network, including the orbitofrontal cortex and striatum (Tang et al. 2012). Similar brain regions were reported in another meta-analysis on food images (van der Laan et al. 2011).
Several studies have investigated how different aspects of food involve this reward network, which is, however, not consistently activated by food images. Obese women exhibited activation of striatum (caudate and putamen), but individuals with normal weight did not (Rothemund et al. 2007). In another study, both obese and normal-weight women exhibited activations of reward areas due to food images (as compared to cars), but the effects were stronger in obese women (Stoeckel et al. 2008). Food odors appear to be distinct from other odors, as processing speed in humans appears to be strongly influenced by the edibility of the odor source (Boesveldt et al. 2010). Indeed, differences in processing of odors related to food and non-food are observable with modern neuroimaging techniques. Odors of the desired foods induced a significantly larger BOLD (blood oxygenation level-dependent) response than odors unrelated to food in the reward-processing areas (such as the ventral tegmental area, ventral striatum, and medial frontal cortex) of ten hungry women (five of which were obese and five were lean) (Bragulat et al. 2010). After stimulation with sweet food odors (chocolate cookie and strawberry/cream) and sweet non-food odors (rose and lilac), the perceived sweetness of the food odors correlated significantly with the activity in the left insula but not the perceived sweetness of the flower odors. This result indicates that some characteristics of food odors are also processed in the gustatory cortex, which seems not be the case for non-food odors (see also Seo et al. 2013). The authors, however, did not report any differences in activation patterns between food and non-food odors (Veldhuizen et al. 2010). In another report, participants smelled food odors (pasta and roast beef) and a non-food odor (Douglas fir). Food odors evoked higher activities in reward areas than the non-food odor, such as in the anterior cingulate and medial prefrontal cortex (Eiler et al. 2012). The activation of reward areas by food seems not to be specific for humans, since rats show similar patterns. In fact, exposure to the food odor benzaldehyde (almond) led to activations of structures related to the limbic system of rats, as opposed to three other odors (Kulkarni et al. 2012).
In the present study we set out to investigate differences in brain activity evoked by pleasant food and non-food odors. Although the reward system is multifaceted (Berridge and Robinson 2003), still the major neurotransmitter modulating reward is dopamine (Volkow et al. 2011). Because this system is so well characterized, a particular emphasis was put on the mesolimbic dopaminergic pathway of the brain, i.e., ventral tegmental area (VTA), nucleus accumbens, striatum, amygdala, prefrontal cortex, and anterior cingulate (Volkow et al. 2011; Arias-Carrion et al. 2010). For these areas, we expected differential activations following stimulation with food odors than with non-food odors. Other than the most previous studies, we investigated a population with normal body weight and did not introduce the variable of “hunger” (subjects were expected to be not hungry during the scans). Our particular emphasis was to isolate the variable “food” by comparing food vs non-food odors of similar intensity and pleasantness.
Methods
Ethics Statement
The study was conducted according to the Declaration of Helsinki and was approved by the Ethics Board of the medical faculty of the TU Dresden (application number EK155052012). All participants gave written informed consent before commencing the study.
Participants
A total of 35 participants [14 men, 21 women, 28.0 (standard deviation 9.4) years] were screened. Of these, we selected 23 [7 men, 16 women, 26.9 (7.2) years] for participation in the experiment. Inclusion criteria were (1) normal sense of smell and (2) positive evaluation of the used odorants.
Normal olfactory function was ascertained by the well-established and validated “Sniffin’ Sticks” test (Hummel et al. 1997). This test is based on pen-like odor dispensing devices and allows for the separate evaluation of odor threshold, odor discrimination ability, as well as odor identification ability. The scores of these subtests can be combined to a sum score, which can be compared with normative data (Hummel et al. 2007). We further assessed the participants’ odor perception by exposing them to bottles containing the six odors of strawberry, orange, mango, lily of the valley, jasmine, and lavender. We asked the subjects to evaluate these odors using an 11-step visual analog scale with −5 (very unpleasant), 0 (neutral), and 5 (very pleasant) as anchors. Only subjects who evaluated these odorants above neutral (>0) were included in the study. By doing so we ascertained that the odors which were eventually used in the scanner were not novel for participants.
We further excluded subjects with known olfactory disorder, acute rhinitis, and known neurological or psychiatric diseases.
Materials
In a pilot study we presented a total of 12 odors to 35 participants. These odors included six food odors (chocolate, mango, peach, apple crumble, strawberry, and orange) as well as six non-food odors (rose, cedar, pine, jasmine, lavender, and lily of the valley (all Takasago, Paris, France). Participants rated pleasantness and edibility of each odor by means of visual analog scales ranging from 0 to 10. We selected three odors of both categories by using the following criteria: (1) odors had to be perceived as pleasant, as indicated by an average pleasantness of >5; (2) food odors had to be rated as edible, as indicated by an average edibility rating of >5; (3) non-food odors had to be rated as non-edible, as indicated by an average edibility rating of <5.
Of the odors fulfilling these criteria we selected those with the smallest standard deviation on both variables. This resulted in six odors, namely, strawberry (S), orange (O), mango (M), lily of the valley (V), jasmine (J), and lavender (L) (all Takasago, Paris, France) which were used in the study. Odors were used in neat concentrations.
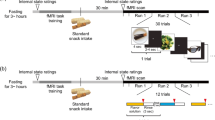
Odor stimulation was performed using a computer-controlled olfactometer (Sommer et al. 2012). In short, this device allows for computer-controlled odor delivery with steep stimulus onset and offset within the scanner. During the stimulation period, odors were delivered to both nostrils in pulses of 1 s, with 2 s between two pulses. Odors were delivered with a flow rate of 1 L/min per nostril. The flow of air was not perceived by the subjects. In the no odor condition, no air was delivered to the participants. Subjects practiced velopharyngeal closure in order to avoid sniffing behavior during the scans (Kobal 1981).
We acquired functional images on a 1.5 T scanner (Siemens Sonata) with the following parameters: EPI (echo planar imaging) sequence, repetition time 2500 ms, echo time 40 ms, flip angle 90°, matrix 64 × 64, voxel size 3 × 3 × 3.75 mm. Subjects were stimulated in an on-/off-block design. They underwent six runs during each of which they were stimulated with only one odor. The order of presentation of the odors was pseudorandomized and counterbalanced. Within one run, participants received six on-blocks and six off-blocks, with each of these blocks lasting 20 s. We were therefore able to acquire, within each run, 48 volumes during on-blocks and 48 volumes during off-blocks.
For overlays we also acquired anatomical T1 scans (magnet-prepared rapid gradient echo (MP-RAGE); voxel size 1 × 0.73 × 0.73 mm). This data was used as reference images in fMRI analysis to account for the poor resolution of functional scans. We chose this design as we had the same paradigm in a number of previous studies where it appeared to work, e.g. (Wallrabenstein et al. 2013; Bensafi et al. 2014; Milinski et al. 2013).
Design
We used a 3 (odors) × 2 (food–flower) repeated measures design.
Procedure
The subjects came to the laboratory for the screening session. After they signed the consent form, their sense of smell was evaluated using the Sniffin’ Sticks. Further, we evaluated their subjective evaluation for six different odorants. Further we verified the study exclusion criteria as well as the MRI exclusion criteria. This lasted for approximately 1 h.
The subjects who passed the screening were invited for participation in the study on another day. The time of testing was between 8 a.m. and 8 p.m. After arriving at the MRI department, the subjects received a short training session and were prepared to enter the scanner room. Once installed in the scanner, we connected the olfactometer outlet to the subjects’ nose. Subjects then underwent the six functional runs. Within each run, they received one single odor; before each run, we told participants the name of the next odor. Importantly, the presentation of different odors was separated by several minutes, in order to avoid cross-contamination. While inside the scanner, participants performed a breathing technique called velopharyngeal closure (Kobal 1981) in order to prevent sniffing in response to odorous stimulation. They were instructed to keep their eyes open.
After each run, subjects evaluated the odor they just had received on three dimensions (pleasantness, edibility, intensity) using 11-point scales (pleasantness, ranging from −5 very unpleasant to 5 very pleasant; edibility, ranging from −5 very inedible to 5 very edible; intensity, ranging from 0 very weak to 10 very intense). In total the functional imaging part lasted for approximately 30 min. Then we acquired the anatomical scan, which lasted another 15 min. In total, the second session lasted approximately 2 h.
Statistical Analysis
Psychophysical data was analyzed by means of SPSS 19.0 (SPSS Inc., Chicago, Il, USA). For descriptive statistics, we calculated average values with standard deviations. We further computed repeated measures ANOVAs for each psychophysical dimension (pleasantness, edibility, intensity) using a 3 (odorants S, O, M and V, J, L) × 2 (quality food, non-food) within subjects design. The alpha level was set at 0.05.
Neuroimaging data was analyzed by means of MatLab (MathWorks, Ismaning, Germany) and the implemented SPM8 toolbox (Wellcome, London, UK). First, we transformed the data from the DICOM format into NifTI format for further analysis. We realigned the volumes to the mean image and coregistered them. Next we segmented the coregistered images into grey and white matter; these files were then used to normalize the realigned images to MNI space. As a last step, we smoothed images with a 7 × 7 × 7 full width at half maximum filter. We then performed a first-level analysis using moving parameters as multiple regressors. We calculated, for each odor and in each subject, a t-contrast between the volumes of the on-blocks and the volumes of the off-blocks, resulting in six t-contrasts. The resulting t-contrasts were used in the second level analysis. Here we calculated the following contrasts: (1) all odors vs baseline, (2) all food odors vs all non-food odors and vice versa, and (3) S vs L and vice versa. We set the significance criterion at p < 0.05, familywise error corrected. For a priori-defined regions, however, we accepted an alpha of p < 0.001, uncorrected, while applying a cluster criterion of >5 voxels.
We further analyzed the data in an additional way. We had a specific hypothesis about the differences between food and flower odors in the VTA. In order to avoid the possibility of false rejections as the result of corrections for multiple comparisons, we refrained from running analyses of additional areas. Accordingly, we extracted the percentage of signal change from the global mean (beta) due to the different odors in this specific brain area. For doing this we used the images obtained in the first level analysis. Coordinates for the VTA were obtained using the Mai brain atlas (Mai et al. 2008); we used the averaged coordinate on each axis resulting in average coordinates of 0, −20, −10 (x, y, z). Using the all odor vs baseline contrast, we automatically searched for the nearest local maximum at the VTA applying a very liberal threshold (p < 0.05, uncorrected). At this location, we extracted the betas for all “On” and “Off” conditions for each odorant. Each of these betas was expressed as the percentage of the session-wide constant beta, yielding the percentage of signal change from the global mean due to the six odorants. We then calculated the difference between the percentage betas for the On and the Off conditions. These values therefore represent the activity in the voxel of the local maximum. We then submitted the resulting values to a repeated measures ANOVA using a 3 (odorants S, O, M and V, J, L) × 2 (quality food, non-food) within subjects design. The alpha level was set at 0.05.
Results
Odors vs Baseline
First, we compared all odors to the baseline. When applying FWE (family-wise error rate) correction, we observed three small clusters located in the posteriolateral extremity of the orbitofrontal cortex, bilaterally, as well as in the posteriolateral prefrontal cortex. See Table 1 for an overview.
Food Odors vs Flower Odors
Psychophysical Data
Participants considered food odors significantly more edible than flower odors; average edibility for food odors ranged from 3.39 to 3.96; average edibility for flower odors ranged from −3.78 to −2.96 (F = 386 (Small et al. 2003; Milinski et al. 2013); p < 0.001). We considered edibility ratings of 1 and more as “edible”; by applying this criterion, 23 (23, 22) of 23 participants rated strawberry (mango, orange) as edible. The one individual rating orange as non-edible assigned it a value of −5. Applying the same criterion for the flower odors, of 23 participants, 0 (2, 0) rated lily of the valley (jasmine, lavender) as edible. The two participants rating jasmine as edible rated it +1. Further, these two did not comprise the individual that misjudged orange. In other words, only in 3 of 138 occasions (6 odors × 23 participants), a participant rated odors into the “wrong” category.
With regards to pleasantness, all odors were considered pleasant, averages ranged between 2.78 (lily of the valley and lavender) and 3.43 (mango); there was no difference between food odors and flower odors. When looking at individual values, ratings were highly consistent. If one considers ratings of 1 and more as pleasant, of 23 participants, 22 (22, 23) rated strawberry (orange, mango) as pleasant. With regards to strawberry, one participant rated it as −1, whereas one subject (not the same individual) rated orange as highly unpleasant (−5). This was the same participants who had rated orange as non-edible. With regards to the flower odors, 23 (23, 22) rated lily of the valley (jasmine, lavender) as pleasant. With regards to lavender, one subject rated its pleasantness as −3. Again, in only 3 of 138 occasions the odor was perceived as unpleasant.
The picture was somewhat more complex with regards to intensity ratings. We observed a significant difference between intensities for food odors and flower odors, despite careful piloting; on average, food odors (averages ranging from 5.52 to 6.65) were perceived as more intense than the flower odors (averages ranging from 4.87 to 5.53; F = 10 (Small et al. 2003; Milinski et al. 2013); p = 0.004). Average ratings are presented in Table 2; see Fig. 1 for an overview overratings.
Average ratings (n = 23) for pleasantness (top), edibility (middle), and intensity (bottom) for six odorants: food odors strawberry (S), orange (O), mango (M), lily of the valley (Li), lavender (La), jasmine (J). The central mark is the median, the edges of the box are the 25th and 75th percentiles; the whiskers extend to the most extreme data points not considered outliers (PP = 1.5 (75th percentile–25th percentile); outliers, >75th percentile + PP or <25th percentile-PP)
Imaging Data
The contrast food vs flower did not reveal any significant differences when FWE correction was applied. A lowered threshold (p < 0.001; extent >5 voxels), however, revealed activations in the left postcentral gyrus and the left superior frontal gyrus (Table 3). In addition to these activations, we further observed a small (2 voxels) activation in the midbrain, 4-mm rostral of the VTA (red circle in Fig. 2a). The inverse contrast showed no significant activations when FWE corrected. When applying the more liberal threshold (p < 0.001, uncorrected with an extent threshold of >5 voxels), we observed two activation clusters, namely, in the left superior frontal gyrus and in the right amygdala.
Differences in brainstem activation between food odors and flower odors. a All three food odors vs all three flower odors [(strawberry + orange + mango) vs (lily of the valley + lavender + jasmine)]. b Strawberry vs lavender (matched for intensity and pleasantness). Encircled area is located in the brainstem
Strawberry vs Lavender
In order to compare iso-intense odors we performed an additional analysis, in which we included only two odorants, namely, strawberry as food odor and lavender as flower odor. Both odors were considered pleasant; there was no difference between this pair of a food odor and a flower odor. Strawberry odor was considered significantly more edible than lavender (p < 0.001). Importantly, both odors had a comparable intensity.
Imaging Data
When comparing strawberry vs lavender, no difference in activations was observed when applying the strict FWE criterion. With the more lenient criterion (p < 0.001, >5 voxels) we observed several significant activations, including the right cingulate, the midbrain (consistent with the ventral tegmental area; red circle in Fig. 2b), and the insula bilaterally. When applying the lenient threshold, the inverse contrast (lavender vs strawberry) revealed significant activations in the hippocampus bilaterally, the right thalamus, and the occipital areas. See Table 4 for details.
Ventral Tegmental Area
We extracted the percentage of signal change from the global mean (beta) for all odorants in all subjects’ VTA. All average betas were significantly above 0 (all p < 0.001). We then computed the repeated measures ANOVA which revealed stronger signal change when subjects smelled food odors compared to flower odors (F = 4.4 (Small et al. 2003; Milinski et al. 2013); p = 0.048). Food odors led to an average signal change in the VTA of 0.20 % (standard error of the mean 0.03); the respective value was 0.14 % (0.02) for flower odors. We next performed post hoc comparisons odor-by-odor for all food-flower combinations. The only two combinations yielding a significant difference between food and flower odor were strawberry vs lavender (p = 0.02) and orange vs lavender (p = 0.009). However, when applying a Bonferroni correction for multiple comparisons, these p-values have to be considered as non-significant. See Fig. 3 and Table 5 for beta values.
Discussion
We observed significant differences in brain activations when the participants were smelling odors related to food and odors related to flowers. The differences were best observable when we only included odors with similar pleasantness and intensity into the analysis (strawberry and lavender). The food odor strawberry activated olfactory processing areas (piriform cortex, insula) and reward/dopaminergic areas (midbrain, cingulate) to a higher degree than the flower odor lavender. The flower odor on the other hand activated memory (hippocampus) and visual areas (cuneus) to a higher degree.
The most significant finding of this current study stems from the comparison between two odors, namely strawberry and lavender. While we interpret this finding as the result of a specific difference between food odors and non-food odors–as we were able to match both odors with regards to intensity and pleasantness–one may argue that the food–non-food dimension is not the only one separating these two odors from each other. In order to generalize our findings, it is indeed necessary to repeat the study with additional matched odors. We tried to do this by using three odors per category, but we found it difficult (and were unable) to match food odors and non-food odors. This may raise the question whether the dimensions we matched them on (intensity and pleasantness) are independent from the edibility dimension or whether intensity and pleasantness are intrinsic contributors to an overall edibility concept. Our study is therefore only the first step to a thorough understanding of how food odors are processed in the brain.
Several studies have shown differences in olfactory processing for food-related odors. In one study, for example, researchers asked healthy young subjects to indicate as fast as possible the presence of an odor. They stimulated their participants with four different odors, two of which were food related and two were not. In both groups of odors, one odor was pleasant, and the other was unpleasant. The authors of this study described that both pleasantness and edibility of the odors affected response times, with the unpleasant food odor evoking shortest detection times (Boesveldt et al. 2010). This finding suggested (negative) food odors to be ecologically more relevant and therefore processed more quickly, in analogy to the visual system (Mineka and Ohman 2002). Another study shed light on possible neuroanatomical properties of food odor processing. Again, researchers presented their participants with four different odorants while they were recording fMRI. Participants received three non-food odors and a food-related odor (chocolate). Further, the researchers stimulated participants via a tubing either ending at the nostril (orthonasal stimulation) or in the nasopharynx (retronasal stimulation (Heilmann and Hummel 2004)). In vivo, retronasal stimulation is occurring during eating and drinking, when molecules are released from the odor source in the oral cavity. Chocolate, but not the three other control odors, evoked differential activations between orthonasal and retronasal stimulation (Small et al. 2005), further indicating a privileged processing of food-related odors. In another particularly interesting study, researchers fed hungry participants chocolate beyond satiety while they were recording PET scans. By doing so, they could investigate the effect of being exposed to the same food in different internal states, ranging from pleasure to aversion. In the hungry condition participants were highly motivated to eat chocolate and exhibited activations of reward areas, including caudomedial OFC (orbitofrontal cortex), striatum, and midbrain. When participants ate chocolate despite being satiated, they evaluated it as less rewarding and showed changed activation patterns (caudolateral OFC, prefrontal regions). Cingulate activation was independent from reward associated with chocolate consumption (Small et al. 2001). Another study further supported the notion of a satiety-dependent activation in the OFC (O'Doherty et al. 2000).
In fact, several studies have reported activations of reward areas in the brains of food-smelling hungry individuals. For example, in one study participants were food deprived for 24 h and highly craved food. They exhibited stronger activations of the limbic system and reward areas, most prominently ventral tegmental area and ventral striatum, when they smelled food-related odors compared to other odors not related to food (Bragulat et al. 2010). The same group investigated another set of odors and confirmed prefrontal, insular, and orbitofrontal activations to food odors but not to non-food odors in hungry individuals (Eiler et al. 2012). The picture is somehow different in studies testing non-hungry individuals: in one report, participants who were not hungry failed to show differences in brain activations between sweet food-related odors and sweet non-food odors. However, insular activation could be observed following stimulation with all odors. For the food odors, ratings of sweetness perception evoked by the odors were correlated with the activity in the insula but only for the food-related odors, not for the other odors (Veldhuizen et al. 2010). This finding lends further support to the notion of a privileged processing of food-related odors in the brain. Our observation with larger activations in the insula, the cingulate, and the midbrain following stimulation with food-related odors is in line with this body of research. The fact that we did not observe orbitofrontal differences may indicate that for our subjects, who were neither hungry nor satiated to the odors we used, both sets of odorants were–to some degree–equally rewarding. However, results from the dopaminergic areas suggest differential activations in these areas depending on the fact whether the stimulus is related to food or not.
The VTA is a central area in the processing of rewarding stimuli. In fact, its dopaminergic neurons are sensitive to the reward prediction error, which is the discrepancy between expected and actual reward (Bayer and Glimcher 2005). After olfactory stimuli had been associated with rewarding or punishing outcomes for rats, 50 % of investigated VTA neurons–all dopaminergic–exhibited activation patterns which followed closely both reward-predicting odors and actual reward. The other half of the neurons–all GABAergic–showed different activation patterns, consistent with a persistent excitation or inhibition between reward-predicting odors and actual reward (Cohen et al. 2012). This particular study is interesting in the present context. First, it provides a neurophysiological underpinning for reward activations and second, it shows that odors can act as reward-predicting stimuli. The final link between reward, the VTA, and food odors is provided by studies which show that food consumption leads to activation of dopaminergic neurons in the VTA: Ghrelin is a hormone which is released by the stomach and induces a rapid orexigenic response (Asakawa et al. 2001). Importantly, it is involved in the reward induced by palatable food (Egecioglu et al. 2010). Ghrelin receptors are located in several brain areas including the hypothalamus, the brainstem, the tegmentum, and the hippocampus (Guan et al. 1997; Zigman et al. 2006). In fact, Ghrelin injection leads to activation of mesolimbic reward areas, such as the VTA and the nucleus accumbens (Egecioglu et al. 2010) and increases the motivation for chemosensory reward (Skibicka et al. 2011). These studies show that both food consumption and the perception of odors associated with food may lead to a strong activation of dopaminergic brain areas. Our results are in line with these conclusions in that a food odor led to higher activity in the VTA than a flower odor matched for intensity and pleasantness. Food-related odors would lead to the activation of dopaminergic areas via association. In future studies it could be investigated to what extent food-related activations of the VTA are hard wired or learned by using odors of unknown food via classical conditioning.
An interesting finding is the activation of regions such as the thalamus, hippocampus, and occipital regions by the flower odors, particularly with lavender. Thalamic activation by lavender may very well be caused by its slight trigeminal component, which may lead to activation of the somatosensory pathway. The hippocampal and the occipital activations are more puzzling. Occipital activation has been reported to occur during odor perception (Djordjevic et al. 2005), but it is unclear why this should be more prominent with lavender than with strawberry. Together with the larger activation of hippocampal activations, one may speculate that lavender evoked visualized memories in the participants, but this is far from supported by the data.
There are limitations to the present study. We did observe perceptual differences between both odor categories. While we tried to control this (by piloting and exclusion of some odors), it may very well be that these dimensions are an integral part of the odor categories. In other words, a food odor per se may be perceived as more or less pleasant as a flower odor at equal intensities; therefore, when trying to match pleasantness of the odors, food odors had to be more intense than flower odors. If this is the case, it would be impossible to perfectly match both odor categories on these dimensions. Also, in the present study odor intensity was not perfectly matched which may have impacted on the results; however, this was not the case for strawberry as food odor and lavender as flower odor. In any case, the results have to be interpreted with some caution as ideal conditions for the analysis were not present for all possible odor pairings. Further, we did not control systematically for hunger–although none of the subjects was starving. Another limitation is that the main effects seen in fMRI data are restricted to the pair strawberry–lavender. Future studies are needed to show whether the observed effect also generalizes to other odor qualities. In terms of technical details, future studies should also include intensity ratings as a covariate in the full analyses.
References
Arias-Carrion O, Stamelou M, Murillo-Rodriguez E, Menendez-Gonzalez M, Poppel E (2010) Dopaminergic reward system: a short integrative review. Int Arch Med 3:24
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T et al (2001) Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120:337–345
Bayer HM, Glimcher PW (2005) Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 47:129–141
Bensafi M, Croy I, Phillips N, Rouby C, Sezille C et al (2014) The effect of verbal context on olfactory neural responses. Hum Brain Mapp 35:810–818
Berridge KC, Robinson TE (2003) Parsing reward. Trends Neurosci 26:507–513
Boesveldt S, Frasnelli J, Gordon AR, Lundstrom JN (2010) The fish is bad: negative food odors elicit faster and more accurate reactions than other odors. Biol Psychol 84:313–317
Bragulat V, Dzemidzic M, Bruno C, Cox CA, Talavage T et al (2010) Food-related odor probes of brain reward circuits during hunger: a pilot FMRI study. Obesity (Silver Spring) 18:1566–1571
Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N (2012) Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482:85–88
Djordjevic J, Zatorre RJ, Petrides M, Boyle JA, Jones-Gotman M (2005) Functional neuroimaging of odor imagery. Neuroimage 24:791–801
Egecioglu E, Jerlhag E, Salome N, Skibicka KP, Haage D et al (2010) Ghrelin increases intake of rewarding food in rodents. Addict Biol 15:304–311
Eiler WJA, Dzemidzic M, Case KR, Considine RV, Kareken DA (2012) Correlation between ventromedial prefrontal cortex activation to food aromas and cue-driven eating: an fMRI study. Chemosens Percept 5:27–36
Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD et al (1997) Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48:23–29
Heilmann S, Hummel T (2004) A new method for comparing orthonasal and retronasal olfaction. Behav Neurosci 118:412–419
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) “Sniffin' sticks”: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52
Hummel T, Kobal G, Gudziol H, Mackay-Sim A (2007) Normative data for the “Sniffin' Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 264:237–243
Kobal G (1981) Elektrophysiologische Untersuchungen des menschlichen Geruchssinns. Thieme, Stuttgart
Kulkarni P, Stolberg T, Sullivanjr JM, Ferris CF (2012) Imaging evolutionarily conserved neural networks: preferential activation of the olfactory system by food-related odor. Behav Brain Res 230:201–207
Mai JK, Paxinos G, Voss T (2008) Atlas of the human brain, viiith edn. Elsevier/Academic, Amsterdam, 271 p
Milinski M, Croy I, Hummel T, Boehm T (2013) Major histocompatibility complex peptide ligands as olfactory cues in human body odour assessment. Proc Biol Sci 280:20122889
Mineka S, Ohman A (2002) Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biol Psychiatry 52:927–937
O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F et al (2000) Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport 11:893–897
Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R et al (2007) Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37:410–421
Seo HS, Iannilli E, Hummel C, Okazaki Y, Buschhuter D et al (2013) A salty-congruent odor enhances saltiness: functional magnetic resonance imaging study. Hum Brain Mapp 34:62–76
Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL (2011) Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 180:129–137
Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M (2001) Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 124:1720–1733
Small DM, Jones-Gotman M, Dagher A (2003) Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 19:1709–1715
Small DM, Gerber JC, Mak YE, Hummel T (2005) Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 47:593–605
Sommer JU, Maboshe W, Griebe M, Heiser C, Hormann K et al (2012) A mobile olfactometer for fMRI-studies. J Neurosci Methods 209:189–194
Stoeckel LE, Weller RE, Cook EW 3rd, Twieg DB, Knowlton RC et al (2008) Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 41:636–647
Tang DW, Fellows LK, Small DM, Dagher A (2012) Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav 106:317–324
van der Laan LN, de Ridder DT, Viergever MA, Smeets PA (2011) The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage 55:296–303
Veldhuizen MG, Nachtigal D, Teulings L, Gitelman DR, Small DM (2010) The insular taste cortex contributes to odor quality coding. Front Hum Neurosci 4:58
Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M et al (2002) “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 44:175–180
Volkow ND, Wang GJ, Baler RD (2011) Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 15:37–46
Wallrabenstein I, Kuklan J, Weber L, Zborala S, Werner M et al (2013) Human trace amine-associated receptor TAAR5 can be activated by trimethylamine. PLoS ONE 8:e54950
Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494:528–548
Acknowledgments
This study was supported by Takasago Europe Perfumery Laboratory SARL. Johannes Frasnelli holds a research chair at the University of Québec à Trois-Rivières and is supported by the Research Centre of the Hôpital du Sacré-Coeur de Montréal.
Compliance with Ethical Standards
ᅟ
Conflicts of Interest
None of the authors declares a conflict of interest.
Research Involving Human Participants
The research protocol was approved by the Ethics Committee of the Medical Faculty of the Technical University of Dresden (TU Dresden) (application number EK155052012).
Informed Consent
All participants provided written informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frasnelli, J., Hummel, C., Bojanowski, V. et al. Food-Related Odors and the Reward Circuit: Functional MRI. Chem. Percept. 8, 192–200 (2015). https://doi.org/10.1007/s12078-015-9193-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-015-9193-8