Abstract
Introduction
It has been suggested that disgust evolved as an emotion that motivates the rejection of rotten and poisonous food. Core disgust is experienced primarily in relation to the sense of taste, and bitterness is an indicator of potential food toxicity. The purpose of the present two studies was therefore to test whether the personality traits disgust proneness (DP, tendency to experience disgust) and disgust sensitivity (DS, tendency to expect harmful consequences of experiencing disgust) are associated with the intensity of perceived bitterness and disgust during the tasting of bitter herbs (e.g., dandelion, wormwood).
Method
Bitter and neutral compounds were presented as dried powder (study 1) or as teas (study 2) to a total of 170 women with a mean age of 23.5 years.
Results
In both experiments, women with high DS reported to experience more disgust when tasting the bitter compounds, but they did not differ in their bitterness ratings from women with low DS scores. DP did influence neither disgust nor bitterness ratings.
Conclusions
Trait disgust was not associated with the sensory perception of bitterness, but with its affective evaluation, the experienced disgust.
Implication
High DS might be considered a hypersensitive alarm system to aversive taste.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The etymological origin of the word “disgust” implies that something has a bad taste (Rozin, Haidt, and McCauley 2008). Bioevolutionary approaches describe disgust as a basic emotion that evolved from the oral rejection of unpleasant taste as a defense mechanism against the ingestion of rotten and poisonous food (Darwin 1965 [1872]; Rozin and Fallon 1987). In line with this conception is the typical facial expression of disgust (Ekman and Friesen 1975). It is composed of the raising of the upper lip and the protrusion of the tongue, which have been interpreted as a vestige of the gag reflex (Rozin and Fallon 1987). The facial disgust expression can be seen as functional in rejecting health-threatening food, and the most distinct somatic concomitant of disgust “nausea” inhibits ingestion. Disgusting objects should not enter the body and therefore need to be spit out. Hence, disgust first evolved to motivate food rejection and later on expanded to other domains (Rozin, Haidt, and McCauley 2008).
According to this definition of disgust, the sense of taste is of critical importance for this basic emotion. Especially, bitter tastes are able to elicit the typical facial display of disgust. It has been suggested that bitterness is an indicator of food toxicity. Many poisons, such as secondary plant metabolites and rancid fats, do taste bitter (Glendinning 1994). Therefore, the detection of bitterness has an important health-protective function. “Bitter” is an alarm signal which helps to prevent the ingestion of these toxic compounds (Meyerhof, Behrens, Bufe, and Kuhn 2005).
The ability of humans to taste bitterness depends on the binding of bitter compounds to bitter taste receptors. These receptors can be found on the surface of the taste receptor cells of the tongue and are encoded by a large family of receptor genes (25 functional genes) named TAS2Rs (Wang, Thomas, and Zhang 2004). The mentioned receptors show genetic variation. For example, the ability to taste the bitter chemical 6-n-propylthiouracil (PROP) depends on the polymorphism of the TAS2R38 gene. The version determines whether one is a “supertaster”, who is highly sensitive to bitterness, a “taster” with moderate sensitivity, or a “nontaster” (Bartousek, Duffy and Miller 1994).
Although an association between bitterness sensitivity and disgust proneness seems logical, there are only a few studies on this topic. Herz (2011) revealed a positive correlation between the two traits. She was able to show that participants who were very sensitive to the bitter-tasting PROP compound (supertasters) were more prone to visceral disgust than PROP nontasters. Similarly, in a study by Herbert et al. (2014), PROP tasters reported higher disgust proneness toward body products than nontasters.
There are many plants that contain bitter compounds in varying degrees. These bitter substances are of different chemical origins and elicit bitter perceptions with different intensities (Hänsel and Sticher 2010). The bitterness intensity of a certain compound is indexed by the bitterness value which is defined as “the reciprocal of the dilution of a compound, a liquid or an extract that still has a bitter taste. It is determined by comparison with quinine hydrochloride, the bitterness value of which is set at 200,000” (Ph. Eur., p. 221; Council of Europe, 2005).
We conducted two studies in order to test participants’ gustatory and affective judgments of bitter compounds. We investigated whether the degree of disgust proneness (tendency of a person to experience disgust across different situations; Schienle, Walter, Bertram, Stark, Rudolf, and Vaitl 2002) would be associated with the bitterness perception and affective evaluation of herbs with varying bitter intensities, served as teas or in dried powdered form. Moreover, we looked at a second disgust-related personality trait, disgust sensitivity, which refers to the perceived harmful consequences of experiencing disgust. Individuals with high scores on disgust sensitivity scales experience their disgust symptoms as more negative and uncontrollable than individuals with low scores (Schienle, Dietmaier, Ille, and Leutgeb 2010).
It was hypothesized that the intensity of perceived bitterness, disgust, valence, and arousal of bitter relative to neural compounds would be associated with measures of disgust proneness and disgust sensitivity.
Study 1
Method
Participants
Sixty-five female university students with a mean age of M = 22.38 years (SD = 2.82) participated in this study. We only tested women because there are significant sex differences in trait disgust (Schienle et al. 2002). Exclusion criteria consisted of somatic disease, medication, vegetarian diet, and cigarette smoking. The women received course credit for their participation. All participants were carefully instructed and gave written informed consent. The study had been approved by the ethics committee of the University of Graz.
Stimuli
Stimuli were dried dandelion root, artichoke leaves, and wormwood as well as a neutral control substance (flour). These stimuli were selected since they cover a wide range of bitterness values. Taraxaci radix cum herba (dandelion root) contains the bitter compound taraxacin; its bitterness value is approximately 100 (Austrian Pharmacopoeia (ÖAB), Bisset 1994). The bitter compound of the artichoke is called cynarin; its bitterness value has a maximum of 11,500 (Weiss and Fintelmann 1999). Absinthii herba (wormwood) mainly contains absinthin. Its bitterness value is defined as at least 10,000 (ÖAB, Bisset 1994). All bitter compounds of the plants are different derivates of the terpene group, the primary components of essential oils. The herbs were provided by a local pharmacy.
Questionnaires
The participants completed a medical checklist by the authors in order to check the exclusion criteria, as well as the Questionnaire for the Assessment of Disgust Proneness (QADP; Schienle et al. 2002) and the Scale for the Assessment of Disgust Sensitivity (SADS; Schienle et al. 2010) via an online survey.
The QADP measures disgust propensity and describes 37 situations, which have to be judged on a five-point scale with regard to the experienced disgust (0 = “not disgusting”; 4 = “very disgusting”). The five subscales are as follows: (1) death/deformation (e.g., “Accidentally you touched the stump of an arm-amputated man”), (2) body secretions (e.g., “Someone intensively smelling of sweat takes the seat beside you in the bus”), (3) spoilage (e.g., “You are just about to drink a glass of milk as you notice that it is spoiled”), (4) poor hygiene (e.g., “You touch the toilet seat with part of your body in a public restroom”), and (5) oral rejection (e.g., “you smell vomit”). The Cronbach’s alpha of the total scale is 0.90.
The SADS consists of seven items addressing the appraisal and control of one’s own disgust feelings (e.g., “Experiencing disgust is stressful for me”; 0 = “strongly disagree”; 4 = “strongly agree”). The Cronbach’s alpha of the scale is 0.85.
Procedure
The taste experiment was scheduled within the next week of the online survey. All substances were presented in powdered form (amount, 1 tsp) to the blindfolded participants. After the tasting, the participants rated the experienced disgust (“How disgusting tasted the substance?”) and the bitterness (“How bitter was the substance?”) on a five-point Likert scale (1 = “not disgusting/bitter”; 5 = “very disgusting/bitter”). There was no time restriction for the evaluation. The stimuli were presented in random order. After each stimulus, the participants rinsed their mouth with water. Finally, they were asked whether they were able to name the substance, which was not possible for any of the participants.
Analysis
We computed analyses of variance with the between-subjects factor group (high vs. low disgust proneness/disgust sensitivity) and the within-subjects factor compound (flour, wormwood, artichoke, and dandelion) for ratings of bitterness and disgust. The group factor had been determined by median split. If violations of sphericity occurred, Greenhouse-Geisser corrections were used. Significant effects were followed up by t tests.
Results
Questionnaires
The women obtained a mean QADP score of M = 2.30 (SD = 0.61) and a mean SADS score of M = 0.86 (SD = 0.62), which does not differ from the values of the female construction samples.
Analyses of Variance
Experienced Disgust
The analyses of variance for the comparison of women with high and low disgust sensitivity (DS) revealed a significant main effect for compound (F(1, 63) = 163.20, p < 0.001, ƞ 2 p = 0.721). The post hoc t tests indicated that wormwood was rated as the most disgusting compound followed by artichoke, dandelion, and flour (all paired comparisons: p < 0.001). We also obtained a significant interaction effect for DS × compound (F(3, 189) = 4.83, p = 0.032, ƞ 2 p = 0.071). Disgust-sensitive women experienced more disgust when tasting dandelion, artichoke, and wormwood (all ps < 0.01), but not flour (Fig. 1).
The analyses of variance for the comparison of the two disgust proneness (DP) groups revealed no significant results except for the compound effect (p < 0.001).
Bitterness
The analyses of variance for the comparison of women with high and low disgust sensitivity (DS) revealed a significant main effect for compound (F(1, 63) = 450, p < 0.001, ƞ 2 p = 0.872). Wormwood had been rated as the most bitter compound followed by artichoke, dandelion, and flour (all paired comparisons, p < 0.001, see Fig. 1). The DS effect was marginally significant (F(1, 63) = 2.28, p = 0.071, ƞ 2 p = 0.036), and the interaction was nonsignificant.
The other analysis of variance with DP as group factor revealed no significant results except for the compound effect (p < 0.001).
The averaged bitterness ratings (across all compounds) and averaged disgust ratings were positively correlated with each other (r = 0.59; p < 0.001).
Study 2
This study was conducted in order to replicate findings of study 1 and to improve certain aspects of the experimental design. First of all, we changed the presentation of the stimuli. Instead of dried powder, we brewed teas. Moreover, the participants were asked to additionally rate the valence and experienced arousal during the taste experiment and to give their judgments on a nine-point instead of five-point scale in order to improve the sensitivity of the measurement.
Method
Participants
A total of 105 women completed the study. Their age ranged from 18 to 49 years (M = 25.42, SD = 6.91). Seventy-six percent of the participants had graduated from high school, and 23 % of the participants had a university degree. Exclusion criteria consisted of somatic disease, medication, vegetarian diet, and cigarette smoking. All participants were carefully instructed and gave written informed consent. The study had been approved by the ethics committee of the University of Graz.
Stimuli
We used five bitter herbs: absinthii herba, centaurii herba, angelicae radix, calami radix, and taraxaci radix, which had been obtained from a local pharmacy. Similar to study 1, all bitter substances were derivates of the terpene group. Absinthii herba (wormwood) and taraxaci radix (dandelion root) have been described above. As new compounds, we studied centaurii herba (lesser centaury), which contains the bitter substance centrapricin. Its bitterness value is defined as at least 2000 (ÖAB, Bisset 1994). Angelicae radix (angelica root) and calami rhizome (calamus) taste bitter because of bitter compounds in essential oils (ÖAB: not less than 0.3 % (angelica root) and 2 % (calamus); Bisset 1994).
The five herbs which did not contain bitter compounds (neutral teas) were alchemillae herba (lady’s mantle), melissae folium (balm), calendulae flos (marigold), rubi idaei folium (raspberry leaf), and malve folium (mallow leaf).
Questionnaires
The participants completed questionnaires for the assessment of DP and DS (QADP; SADS: Schienle et al. 2002, 2010).
Procedure
The teas were tasted blindfolded in randomized order. Each tea was dispensed in the mouth via a glass pipette (20 ml) and left there for 10 s until the participants were allowed to spit it out. All teas were prepared in the same manner: each tea was made with 1 tsp of dried herbal powder per 100 ml of water. The teas steeped for exactly 7 min and then cooled down to room temperature.
After tasting each tea, the participants rated experienced valence (“How pleasant was the tasting of the tea?”), arousal (“How aroused did you feel while tasting the tea?), bitterness (“How bitter did the tea taste?”), and disgust (“How intense did you feel disgust while tasting the tea?”) on a nine-point Likert scale (9 = very pleasant, aroused, very bitter, very disgusting). Before tasting the next tea, the mouth was rinsed with a hydrogen peroxide water solution (1:10 dilution).
Data Analysis
The ratings for valence, arousal, bitterness, and disgust were averaged across the five bitter and the five neutral teas. Post hoc participants were divided into two groups of either high or low DP as well as high or low DS. Eight 2 × 2 ANOVAs were separately conducted for the four ratings with compound as within-subjects factor (bitter vs. neutral) and disgust proneness and disgust sensitivity (DP, DS) as between-subjects factor. For post hoc comparisons, t tests were calculated.
Results
Questionnaires
The participants obtained a mean QADP score of M = 2.24 (SD = 0.60) and a mean SADS score of M = 0.95 (SD = 0.60).
Experienced Disgust
When comparing women with high vs. low disgust sensitivity (DS), the effects for compound (F(1, 103) = 230.62, p < 0.001, ƞ 2 p = 0.69) and DS (F(1, 103) = 5.96, p = 0.02, ƞ 2 p = 0.05) and the interaction DS × compound were significant (F(1, 103) = 5.53, p = 0.02, ƞ 2 p = 0.05). The post hoc t tests indicated that bitter teas were rated as more disgusting than neutral ones (p < 0.001). Women with high DS scores rated the bitter teas as more disgusting than low scorers (p = 0.01). The two DS groups did not differ in their ratings of the neutral teas.
The analyses of variance for the comparison of women with high and low DP revealed significant effects for compound (F(1, 103) = 228.36, p < 0.001, ƞ 2 p = 0.69) and the interaction DP × compound (F(1, 103) = 5.67, p = 0.04, ƞ 2 p = 0.05). The post hoc t tests for the comparison of women with high and low DP scores for bitter teas were marginally significant (p = 0.06).
Experienced Valence, Arousal, and Bitterness
Only the main effects for compound reached statistical significance (all ps < 0.001). Bitter teas were rated as less pleasant (M valence = 3.78, SD = 1.01) and more arousing (M arousal = 1.83, SD = 1.21) than neutral teas (M valence = 6.50, SD = 1.09; M arousal = 1.50, SD = 0.79; all ps < 0.01). Also, the perceived bitterness was higher for bitter teas relative to neutral teas (p < 0.001; see Fig. 2).
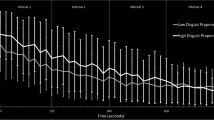
All other effects were nonsignificant (all ps > 0.16) except for the interaction DS × compound with regard to experienced arousal (F(1, 103) = 4.27, p = 0.04, ƞ 2 p = 0.04). Women with high DS scores rated the bitter teas as more arousing than low scorers (p = 0.04).
Given that the bitter tea condition was composed out of five different teas, we conducted additional ANOVAs that included the within-subjects factor compound (wormwood, lesser centaury, angelica root, calamus, and dandelion root) and the between-subjects factor (DS or DP) for disgust and bitterness ratings.
Experienced Disgust
Both ANOVAs yielded a significant main effect for compound (F(3.20, 329.96) = 73.10, p = 0.00, ƞ 2 p = 0.42 and F(3.26, 335.49) = 76.90, p = 0.00, ƞ 2 p = 0.43). The wormwood tea (M = 5.58 (SD = 2.71)) elicited more disgust than lesser centaury (M = 4.99 (SD = 2.70)). The wormwood and the lesser centaury tea elicited more disgust than angelica root (M = 2.64 (SD = 2.1)), calamus (M = 2.42 (SD = 1.92)), and dandelion root tea (M = 2.74 (SD = 2.07) (all ps < 0.05).
Participants with high DS rated the teas as more disgusting than participants with low DS (main effect DS: F(1,103) = 6.62, p = 0.01, ƞ 2 p = 0.06; Fig. 2). The significant interaction effect DS × compound indicated that the disgust ratings were higher for wormwood, lesser centaury, and angelica root tea for high compared to low disgust-sensitive individuals (F(3.26, 335.49) = 3.04, p = 0.03, ƞ 2 p = 0.03; wormwood: t(103) = −2.96, p = 0.01, d = 0.54; lesser centaury: t(103) = −2.60, p = 0.01, d = 0.49; angelica root: t(103) = –1.94, p = 0.05, d = 0. 0.38; see also Fig. 2). No other effect reached statistical significance.
Experienced Bitterness
Both ANOVAs only yielded the significant main effect for compound (F(3.59, 369.70) = 205.72, p = 0.00, ƞ 2 p = 0.67 and F(3.44, 354.72) = 254.16, p = 0.00, ƞ 2 p = 0.71). The wormwood (M = 8.17 (SD = 1.36)) and the lesser centaury tea (M = 7.89 (SD = 1.23)) were experienced more bitter than angelica root (M = 2.84 (SD = 1.98)), calamus (M = 3.51 (SD = 2.24)), and dandelion root tea (M = 3.39 (SD = 2.15) (all ps < 0.05). No other effect reached statistical significance.
The averaged bitterness ratings (across all compounds) correlated with the averaged ratings for disgust (r = 0.45; p < 0.001), valence (r = –0.61, p < 0.001), and arousal (r = 0.25, p = 0.009).
Discussion
One core function of disgust is the protection of the body from food poison (Rozin et al. 2008). As bitterness has been conceptualized as one indicator of food toxicity, we hypothesized that disgust-prone (disgust-sensitive) individuals would be characterized by heightened bitterness sensitivity. The findings of the two conducted studies were not in line with this assumption. Scores on the two trait disgust measures were not associated with the reported intensity of bitterness sensation. Thus, trait disgust did not influence the sensory decoding of bitterness.
However, depending on their habitual disgust reactivity, the participants varied in the experienced disgust during the tasting of the bitter compounds. In studies 1 and 2, high DS predicted a stronger disgust experience and thus a more negative evaluation of the bitter compounds.
DS is rooted in certain beliefs that the experience of disgust is something aversive and stressful, which is additionally difficult to control. Obviously disgust-sensitive individuals do not view disgust as a health-promoting mechanism, but something that implies danger (Schienle et al. 2010). Everybody wants to be safe and avoid disease, but people with high DS place a significantly higher value on their safety. Therefore, it is understandable that they respond to potential indicators of food toxicity such as bitter taste with more disgust as this motivates safety behavior (e.g., food avoidance, food rejection). Chen and Chang (2012) already demonstrated that the sensation of bitter taste was associated with enhanced motivation for survival. They showed that individuals who had tasted a bitter drink responded faster to survival-related words in a lexical decision task than those who drank plain water.
The DS bias observed in our two studies consisted of a different appraisal but was not grounded on a different sensory experience of bitterness. With regard to reducing the risk of potential food poisoning, such a behavior even makes sense as the degree of bitterness is only loosely associated with the toxicity of plant. Glendinning (1994) showed that bitter taste thresholds varied independently of toxicity thresholds across different species. This implies that a bitter rejection response is just as likely to be elicited by a harmless bitter food as it is by a harmful one. Glendinning (1994) suggested that the bitter sensitivity of humans and animals is mainly based upon their typical diet and, consequently, on learning experiences. For example, herbivores with a relatively high amount of bitter compounds in their diet have a higher bitter taste threshold than carnivores. In accordance with this, Duffy et al. (2010) showed that the typical diet of individuals was associated with their perception of bitterness. Subjects who had reported greater consumption of vegetables were less bitter-sensitive.
The elevated disgust reactivity to bitterness by disgust-sensitive individuals could therefore be understood as a hypersensitive warning system. In line with this interpretation are findings by Herbert et al. (2014) who demonstrated a link between bitter sensitivity and general emotional approach-avoidance behavior. The participants (PROP tasters and nontasters) were presented with affective pictures and received an acoustic startle probe. The PROP tasters, who had reported elevated DP, showed facilitated startle eye blink responses during the viewing of affective compared to neutral pictures. Thus, bitter sensitivity predicted emotional reactivity even in a different sensory modality (visual instead of gustatory). Already, Macht and Mueller (2007) had speculated that bitterness (PROP) sensitivity is associated with an increased arousability of emotions such as anger, fear, and disgust.
Our findings showed moderate positive correlations between ratings of bitterness, disgust, and arousal, which is in line with previous reports (e.g., Garcia-Burgos and Zamora 2013, 2015). Moreover, individuals high in DS labeled bitter as more disgusting and arousing. Future studies should investigate whether this bias is based upon previous learning experience and whether this bias changes over time or can be changed by certain interventions. As we age, we lose taste buds, and we also learn that not all bitter foods are bad. In fact, we realize that many bitter foods (e.g., coffee, dark chocolate) stimulate our nervous system and can even protect us against illness (Reed, Tanaka, and McDaniel 2006). Many bitter compounds of plants are considered herbal drugs, which are often used to stimulate digestion and appetite. Hence, a bitter taste does not generally mean that food is poisonous but signalizes that a little amount might even have positive effects.
We have to mention several limitations of our investigation. We only studied women. Therefore, our findings cannot be generalized to men. Also, we should have obtained more detailed information on food intake (type, frequency) in order to include this factor as a moderator into the analysis. We only analyzed responses to a limited number of bitter compounds and did not include additional taste conditions in the design. It is possible that very salty, sour, or even very sweet substances would be rated as more disgusting by disgust-sensitive individuals. This response specificity needs further investigation. Finally, one reviewer of this manuscript pointed out that Likert scales as intensity descriptors have no universal meaning for individuals and are therefore not optimal for group comparisons. Some authors (e.g., Bartoshuk et al. 2005) suggested to express sensations of interest (e.g., bitterness) relative to an unrelated stimulus (e.g., brightness) or to use different anchors for the hedonic scales (e.g., “strongest imaginable (dis)liking of any kind”.). A replication of the present study which only modifies the response format for the rating procedure can provide valuable information on this topic.
References
Bartoshuk LM, Fast K, Snyder DJ (2005) Differences in our sensory world. Invalid comparisons with labeled scales. Curr Dir Psychol Sci 14(3):122–125
Bartoshuk LM, Duffy VB, Miller IJ (1994) PTC/PROP tasting: anatomy, psychophysics and sex effects. Physiol Behav 56(6):1165–1171
Bisset NG (ed) (1994) Herbal drugs and phytopharmaceuticals: A handbook for practice on a scientific basis. Medpharm Scientific Publication, Stuttgart
Chen B-B, Chang L (2012) Bitter struggle for survival: evolved bitterness embodiment of survival motivation. J Exp Soc Psychol 48(2):579–582
Council of Europe (ed) (2005) European Pharmacopoeia: 2815 bitterness value. Council of Europe, Strasbourg
Darwin CR (1965 [1872]) The expression of the emotions in man and animals. London: Penguin
Duffy VB, Hayes JE, Davidson AC, Kidd JR, Kidd KK, Bartoshuk LM (2010) Vegetable intake in college-aged adults is explained by oral sensory phenotypes and TAS2R38 genotype. Chemosens Percept 3:137–148
Ekman P, Friesen WV (1975) Unmasking the face (1 Auflage). Prentice Hall, Eaglewood Cliffs
Garcia-Burgos D, Zamora MC (2013) Facial affective reactions to bitter-tasting foods and body mass index in adults. Appetite 71:178–186
Garcia-Burgos D, Zamora MC (2015) Exploring the hedonic and incentive properties in preferences for bitter foods via self-reports, facial expressions and instrumental behaviours. Food Qual Prefer 39:73–81
Glendinning JI (1994) Is the bitter rejection response always adaptive? Physiol Behav 56(6):1217–1227
Hänsel R, Sticher O (eds) (2010) Pharmakognosie—Phytopharmazie (9 überarbeitete und aktualisierte Auflage). Springer Medizin Verlag, Heidelberg
Herbert C, Platte P, Wiemer J, Macht M, Blumenthal TD (2014) Supertaster super reactive: oral sensitivity for bitter taste modulates emotional approach and avoidance behavior in the affective startle paradigm. Physiol Behav 135:198–207
Herz RS (2011) PROP taste sensitivity is related to visceral but not moral disgust. Chemosens Percept 4(3):72–79
Macht M, Mueller J (2007) Increased negative emotional responses in PROP supertasters. Physiol Behav 90(2-3):466–472
Meyerhof W, Behrens M, Bufe B, Kuhn C (2005) Human bitter taste perception. Chem Senses 30(Supplement 1):i14
Reed DR, Tanaka T, McDaniel AH (2006) Diverse tastes: genetics of sweet and bitter perception. Physiol Behav 88(3):215–226
Rozin P, Fallon AE (1987) A perspective of disgust. Psychol Rev 94(1):23–41
Rozin P, Haidt J, McCauley CR (2008) Disgust. In: Lewis M, Haviland-Jones JM, Barrett LF (eds) Handbook of emotions, 3rd edn. Guilford Press, New York, pp 757–776
Schienle A, Dietmaier G, Ille R, Leutgeb V (2010) Eine Skala zur Erfassung der Ekelsensitivität (SEE) Zeitschrift für Klinische. Psychologie und Psychotherapie 39(2):80
Schienle A, Walter B, Stark R, Vaitl D (2002) Ein Fragebogen zur Erfassung der Ekelempfindlichkeit (FEE) Zeitschrift für Klinische. Psychologie und Psychotherapie 31(2):110–120
Wang X, Thomas SD, Zhang J (2004) Relaxation of selective constraint and loss of function in the evolution of human bitter taste receptor genes. Hum Mol Genet 13(21):2671–2678
Weiss RF, Fintelmann V (1999) Lehrbuch der Phytotherapie (9 Auflage). Hippokrates-Verlag, Stuttgart
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflict of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schienle, A., Arendasy, M. & Schwab, D. Disgust Responses to Bitter Compounds: the Role of Disgust Sensitivity. Chem. Percept. 8, 167–173 (2015). https://doi.org/10.1007/s12078-015-9186-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-015-9186-7