Abstract
Background
The classification and nomenclature of non-alcoholic fatty liver disease (NAFLD) has been the subject of ongoing debate in the medical community. Through the introduction of metabolic dysfunction-associated fatty liver disease (MAFLD) and the later release of metabolic dysfunction-associated steatotic liver disease (MASLD), the limitations associated with NAFLD are intended to be addressed. Both terminologies incorporate the metabolic component of the disease by providing diagnostic criteria that relies on the presence of underlying metabolic risk factors.
Materials and Methods
An epidemiologic cross-sectional study of individuals who had undergone abdominal ultrasound and vibration-controlled transient elastography (VCTE) as part of a routine check was performed. We evaluated clinical, anthropometric, and biochemical variables to determine the metabolic profile of each subject.
Results
The study included a total of 500 participants, 56.8% (n = 284) males and 43.2% (n = 216) females, with a mean age of 49 ± 10 years. 59.4% (n = 297) were diagnosed with MAFLD and MASLD, 10.2% (n = 51) were diagnosed only with MASLD and 30.4% (n = 152) were not diagnosed with either MAFLD or MASLD. The differences in prevalence were mainly based on the detection of individuals with a BMI < 25 kg/m2, where MASLD captures the largest number (p < 0.001).
Conclusions
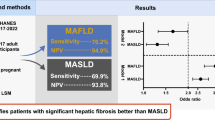
Although MASLD has a higher capture of lean patients compared to MAFLD, patients with MAFLD and MASLD have a worse metabolic profile than those with only MASLD. Our results provide evidence that MAFLD better identifies patients likely to have a higher risk of liver fibrosis and of disease progression.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
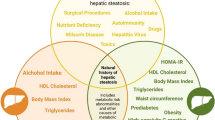
The prevalence of fatty liver disease (FLD) has increased dramatically in recent decades [1]. FLD is considered one of the most common liver disorders worldwide, with a global prevalence of approximately 38% [2]. Since the introduction of metabolic dysfunction-associated fatty liver disease (MAFLD) [3, 4], the renaming of non-alcoholic fatty liver disease (NAFLD) has been a source of debate. To date, the use of MAFLD has proven superior to the use of NAFLD by demonstrating a greater ability to detect individuals with a higher number of risk factors and a higher risk of developing liver fibrosis [5, 6]. Although the advantages of MAFLD are supported by extensive clinical evidence and its use has been supported by stakeholders around the world [7], no agreement has been reached on which terminology is more appropriate [8,9,10]. Recently, the terms steatotic liver disease (SLD) and metabolic dysfunction-associated steatotic liver disease (MASLD) have been proposed, with the same aim as MAFLD, to reduce the limitations associated with the previous terminology [11]. Both terminologies incorporate the metabolic component of the disease (with differences) by providing diagnostic criteria that rely on the presence of underlying metabolic risk factors [4, 11] (Fig. 1).
Overview of MAFLD and MASLD diagnostic criteria. This figure provides a comprehensive visual representation of the diagnostic criteria for Metabolic Associated Fatty Liver Disease (MAFLD) and Metabolic Associated Steatohepatitis (MASLD). It illustrates the key parameters and clinical markers that are considered by each definition
Correct identification of individuals at high metabolic risk is crucial, considering the close association between FLD and other complex metabolic diseases [12]. In this regard, cardiovascular disease (CVD) has been identified as the major cause of mortality in patients with FLD [13]. The association between FLD and CVD is attributable to several factors, including obesity, diabetes mellitus and atherogenic dyslipidemia [14]. Obesity, a prevalent condition in contemporary societies, is also a well-known risk factor for both FLD and CVD. Excessive fat accumulation not only affects the liver, but also causes systemic inflammation and metabolic disturbances, contributing significantly to cardiovascular complications [15]. Both disorders also have insulin resistance (IR) as a pathophysiological contributor. IR promotes inflammation and dyslipidemia, which are key drivers of atherosclerosis, the underlying cause of most CVD [16]. As MAFLD progresses, the risk of cardiovascular complications increases, underscoring the critical need for healthcare professionals to recognize and treat both conditions in a comprehensive and integrated manner to optimize patient care and outcomes [17].
In this setting, the motivation for our study lies in the recognition that the MAFLD and MASLD definitions encompass multifaceted aspects of liver disease associated with metabolic abnormalities. Nevertheless, despite their apparent similarities, subtle differences exist that merit meticulous examination. We conducted the present study with the aim of comparing the use of MAFLD and MASLD and to evaluate the clinical impact of both definitions.
Materials and methods
The publication of this article follows the editorial and methodological recommendations of the STROBE Declaration, the Equator Network and the ICMJE.
Patients
We carried out an epidemiologic cross-sectional study of 500 individuals who had undergone abdominal ultrasound and vibration-controlled transient elastography (VCTE) as part of a routine check at Medica Sur Clinic & Foundation in Mexico City. The study enrolled patients through random selection, with no previous diagnosis of NAFLD, MAFLD, or MASLD.
Data collection
All data were collected from review of the medical records. Clinical evaluation, imaging studies and laboratory test were performed the same day. The following information was obtained: age, sex, systolic and diastolic blood pressure (mmHg), body mass index (BMI) (kg/m2), body fat (%), waist circumference (cm), hip circumference (cm), and waist-hip ratio. The following information was obtained from the laboratory reports: platelets (× 103/μL), creatinine (mg/dL), triglycerides (mg/dL), cholesterol (mg/dL), HDL cholesterol (mg/dL), LDL cholesterol (mg/dL), high sensitivity C-reactive protein (hsCRP) (mg/dL), total bilirubin (mg/dL), glutamic oxaloacetic transaminase (SGOT) (mg/dL), glutamic pyruvic transaminase (SGPT) (mg/dL), alkaline phosphatase (ALP) (mg/dL), gamma glutamyl transferase (GGT) (mg/dL), thyroid stimulating hormone (TSH) (mg/dL), glycosylated hemoglobin (HbA1c) (%).
Diagnosis of fatty liver
The diagnosis of fatty liver by ultrasound was based on the following criteria: heightened contrast between the liver and kidneys, elevated liver parenchyma echogenicity, obscured visualization of intrahepatic vessels, and/or modifications in diaphragm visibility. Liver stiffness (LSM) (kPa) and controlled attenuation parameter (CAP) (dBm) measurements were used for VCTE diagnosis as per manufacturer recommendations using the Fibroscan 502 Touch®.
Diagnosis of MAFLD and MASLD
For the diagnosis of MAFLD, the criteria proposed by the consensus of experts in 2020 was used [18]. The following criteria were considered for diagnosis: evidence of hepatic steatosis, in this case by ultrasound or VCTE, plus one of the following: overweight/obese (BMI ≥ 25 kg/m2) or lean/normal weight (BMI < 25 kg/m2) with evidence of metabolic dysregulation. Metabolic dysregulation was defined as the presence of at least two metabolic risk abnormalities: (a) waist circumference ≥ 102/88 cm in men and women, respectively, (b) systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 85 mmHg, (c) plasma triglycerides ≥ 150 mg/dL, (d) plasma HDL-cholesterol < 40 mg/dL for men and < 50 mg/dL for women, (e) prediabetes (HbA1c 5.7–6.4%) and (f) hs-CRP > 2 mg/dL.
For the diagnosis of MASLD, the criteria proposed by the multi-society consensus in 2023 was used [11]. These include evidence of hepatic steatosis by ultrasound or VCTE, combined with one of the following: (a) BMI ≥ 25 kg/m2 or waist circumference > 94/80 cm in men and women, respectively, (b) HbA1c ≥ 5.7%, (c) systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 85 mmHg, (d) plasma triglycerides ≥ 150 mg/dL and (e) plasma HDL-cholesterol < 40 mg/dL for men and < 50 mg/dL for women.
Statistical analysis
Continuous variables are expressed as mean, median and range. Categorical variables are expressed as frequencies and percentages. ANOVA and chi-square test were used to analyze differences between groups for quantitative and qualitative variables, respectively with p values < 0.05 considered significant. To assess the congruence and consistency of diagnoses between ultrasound and VCTE methods for MAFLD and MASLD, we employed Pearson’s chi-square test and Kappa statistics. A multinomial multivariate model was performed, with the dependent variable being the diagnosis of MAFLD/MASLD.
Results
Population characteristics
In the study sample, 56.8% (n = 284) were male and 43.2% (n = 216) female, with a mean age of 49 years, 95% CI = 48.38 to 50.64, with a median of 50 years. 59.4% (n = 297) were diagnosed with MAFLD and MASLD, 10.2% (n = 51) were diagnosed only with MASLD and 30.4% (n = 152) did not meet MAFLD and MASLD criteria, Table 1 summarizes the baseline characteristics of the population.
Diagnosis of MAFLD and MASLD
There is no significant difference between the diagnosis of MAFLD and MASLD by ultrasound or VCTE, with a substantial level of concordance (K = 0.644, p = 0.000) for MAFLD and with a moderate concordance level (K = 0.490, p = 0.000) for MASLD, confirming the feasibility of using readily available imaging methods to diagnose the disease.
Comparison of the variation in the proportion of subjects with MAFLD and MASLD
Overall the prevalence of MAFLD was 59.4% (n = 297), while the prevalence of MASLD was 69.9% (n = 348).When we applied the different cut-off points for each of the parameters used in the MAFLD and MASLD diagnostic criteria, we observe that the differences in prevalence are mainly based on the detection of individuals with a BMI < 25 kg/m2, where MASLD captures the largest number of lean individuals (p < 0.001) (Table 2).
Metabolic profiling of individuals with MAFLD and MASLD
To assess the metabolic profile of individuals, we evaluated the number of metabolic alterations present in the individuals (Fig. 2) as well as their quantification. Individuals with MAFLD and MASLD have a higher metabolic risk profile compared to individuals who only have a diagnosis of MASLD (Table 3). The difference in metabolic risk profiles observed among these groups highlights the importance of discriminating between the two diagnostic categories.
Multinomial multivariate model
The data in our multinomial mutivariable model (Table 4) suggests that women (compared to men) have 2.3 times the risk of presenting MAFLD and MASLD (OR = 2.30, CI95% = 1.16–4.56); likewise, subjects with an increase in BMI (OR = 2.46, 95%CI = 2.03–2.99) and those with an increase in the percentage of glycosylated hemoglobin (OR = 2.15, 95%CI = 0.80–5.73), have two times the risk of presenting with MAFLD and MASLD. The rest of the variables show a marked underestimation in the effect of the ORs, tending towards a value of no effect. The model allows us to classify 91% of the subjects presenting as MAFLD and MASLD.
Discussion
Previously, the ability of MAFLD to detect individuals with a high-risk metabolic profile and an increased risk of disease progression has been demonstrated [19, 20]. As we demonstrate, while both MAFLD and MASLD criteria require the presence of metabolic dysfunction, an intriguing distinction arises when we consider the number of patients included in each definition. The MASLD definition appears to encompass a larger number of individuals, which translates into a higher prevalence of the disease. This divergence in prevalence raises an important question: does a higher prevalence automatically make one definition superior to the other, especially in the case of a condition as widespread worldwide as FLD? [21, 22]. This forces us to scrutinize the true essence of these definitions and their impact on patient care.
The key to answer this question resides in the ability to precisely identify high-risk individuals. The change from NAFLD to MAFLD was initiated with the overall goal of redefining the disease to better reflect its underlying metabolic nature and to help in risk-stratification [23]. The change in nomenclature was a response to our evolving understanding of the disease, recognizing that metabolic dysfunction is not only associated with the disease, but is in fact intrinsic to it [1, 24,25,26]. Therefore, the primary concern here is not simply the prevalence of the disease, but rather its accuracy and more importantly clinical relevance for identifying those at increased risk of adverse outcomes [5, 20, 27, 28]. We observed that the difference between MAFLD and MASLD is principally localized to the detection of lean individuals; the basis for this is the number of metabolic risk abnormalities required for a diagnosis. For MASLD, lean individuals must have at least one metabolic risk abnormality, while for MAFLD they must have two. This may lead to overdiagnosis or misclassification of individuals without high metabolic risk under the MASLD definition. This comes as no surprise since several studies suggest that NAFLD and MASLD identify identical patient groups (98%) and hence would not be expected to risk stratify patients any better than NAFLD. In turn, this deficiency limits the ability of the MASLD definition firstly to identify homogenous patient groups (3 in the case of MAFLD—overweight/obese, normal weight with two metabolic risk factors and those with type 2 diabetes and at least 5 for MASLD) with different clinical features in cross sectional studies and different disease trajectories, as well as different risks of liver fibrosis [29]. These aspects are critical at the bedside for clinical management.
The main limitation of our study is the sample size as some of the clinical differences between MAFLD and MASLD could not be substantiated by statistical analysis. In addition, the categorization of the dependent variable implies a mismatch between the subgroups of subjects when contrasting the estimators of interest, whether proportions or means. Furthermore, the lack of adequate representation of individuals with MAFLD or MASLD with significant liver fibrosis is a limitation. Consequently, we were unable to explore this critical aspect of the disease in depth, limiting the scope of our conclusions and our insights into the advanced stages of MAFLD and MASLD. These limitations underscore the need for larger and more diverse data sets to reach more robust conclusions about clinical differences and disease progression rates in MAFLD and MASLD.
Conclusion
Both MAFLD and MASLD identify individuals with hepatic steatosis and metabolic dysfunction. Although MASLD encompasses a larger cohort, this should not be a factor that determines selecting one definition over the other. Rather, clinical emphasis should remain on accurately identifying high-risk individuals, consistent with the overall goal of redefining and reclassifying fatty liver disease in the context of its metabolic underpinnings. The use of MAFLD and MASLD definitions have significant implications for clinical practice. Recognizing the differences in diagnostic accuracy between both, health care professionals should be aware of the limitations of each category. This is especially important to avoid over-diagnosis or misclassification of patients at low metabolic risk. Regarding future research, long-term studies are needed to understand how the different subtypes of MAFLD and MASLD progress over time and how they respond to different medical and lifestyle interventions. It is also important to conduct studies to validate and ensure the consistency of the MAFLD and MASLD classification criteria to avoid confusion and ensure the global applicability of this new nomenclature. Future research and clinical practice will undoubtedly provide insight into the utility and implications of these evolving definitions for the management of this globally important health problem.
Data availability
All data supporting the findings of this study are available within the paper.
References
Ramírez-Mejía MM, Qi X, Abenavoli L, Romero-Gómez M, Eslam M, Méndez-Sánchez N. Metabolic dysfunction: the silenced connection with fatty liver disease. Ann Hepatol. 2023;28(6):101138. https://doi.org/10.1016/j.aohep.2023.101138. (Epub 20230717; PubMed PMID: 37468095)
Chan KE, Koh TJL, Tang ASP, Quek J, Yong JN, Tay P, et al. Global prevalence and clinical characteristics of metabolic-associated fatty liver disease: a meta-analysis and systematic review of 10 739 607 individuals. J Clin Endocrinol Metab. 2022;107(9):2691–2700. https://doi.org/10.1210/clinem/dgac321. (PubMed PMID: 35587339)
Eslam M, Alkhouri N, Vajro P, Baumann U, Weiss R, Socha P, et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol. 2021;6(10):864–873. https://doi.org/10.1016/s2468-1253(21)00183-7. (Epub 20210806 PubMed PMID: 34364544)
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. https://doi.org/10.1016/j.jhep.2020.03.039. (Epub 20200408 PubMed PMID: 32278004)
Lim GEH, Tang A, Ng CH, Chin YH, Lim WH, Tan DJH, et al. An observational data meta-analysis on the differences in prevalence and risk factors between MAFLD vs NAFLD. Clin Gastroenterol Hepatol. 2023;21(3):619–29.e7. https://doi.org/10.1016/j.cgh.2021.11.038. (Epub 20211204 PubMed PMID: 34871813)
Yamamura S, Eslam M, Kawaguchi T, Tsutsumi T, Nakano D, Yoshinaga S, et al. MAFLD identifies patients with significant hepatic fibrosis better than NAFLD. Liver Int. 2020;40(12):3018–3030. https://doi.org/10.1111/liv.14675. (PubMed PMID: 32997882)
Méndez-Sánchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7(5):388–390. https://doi.org/10.1016/s2468-1253(22)00062-0. (Epub 20220303 PubMed PMID: 35248211)
Méndez-Sánchez N, Díaz-Orozco LE. Editorial: International consensus recommendations to replace the terminology of non-alcoholic fatty liver disease (NAFLD) with metabolic-associated fatty liver disease (MAFLD). Med Sci Monit. 2021;27:e933860. https://doi.org/10.12659/msm.933860. (Epub 20210712, PubMed PMID: 34248137; PubMed Central PMCID: PMC8284081)
Fouad Y, Elwakil R, Elsahhar M, Said E, Bazeed S, Ali Gomaa A, et al. The NAFLD-MAFLD debate: eminence vs evidence. Liver Int. 2021;41(2):255–260. https://doi.org/10.1111/liv.14739. (Epub 20201202 PubMed PMID: 33220154)
Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. 2021;73(3):1194–1198. https://doi.org/10.1002/hep.31420. (Epub 20210206 PubMed PMID: 32544255)
Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023. https://doi.org/10.1016/j.jhep.2023.06.003. (Epub 20230620. PubMed PMID: 37364790)
Pipitone RM, Ciccioli C, Infantino G, La Mantia C, Parisi S, Tulone A, et al. MAFLD: a multisystem disease. Ther Adv Endocrinol Metab. 2023;14:20420188221145548. https://doi.org/10.1177/20420188221145549. (Epub 20230128. PubMed PMID: 36726391; PubMed Central PMCID: PMC9885036)
Wen W, Li H, Wang C, Chen C, Tang J, Zhou M, et al. Metabolic dysfunction-associated fatty liver disease and cardiovascular disease: a meta-analysis. Front Endocrinol (Lausanne). 2022;13:934225. https://doi.org/10.3389/fendo.2022.934225. (Epub 20220916. PubMed PMID: 36187109; PubMed Central PMCID: PMC9523252)
Targher G. Concordance between MAFLD and NAFLD diagnostic criteria in ‘real-world’ data. Liver Int. 2020;40(11):2879–2880. https://doi.org/10.1111/liv.14623. (PubMed PMID: 32738082)
Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological molecular mechanisms of obesity: a link between MAFLD and NASH with cardiovascular diseases. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms222111629. (Epub 20211027. PubMed PMID: 34769060; PubMed Central PMCID: PMC8583943)
Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. https://doi.org/10.1016/j.metabol.2021.154766. (Epub 20210322 PubMed PMID: 33766485)
Targher G, Corey KE, Byrne CD. NAFLD, and cardiovascular and cardiac diseases: Factors influencing risk, prediction and treatment. Diabetes Metab. 2021;47(2):101215. https://doi.org/10.1016/j.diabet.2020.101215. (Epub 20201206. PubMed PMID: 33296704)
Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999-2014.e1. https://doi.org/10.1053/j.gastro.2019.11.312. (Epub 20200208 PubMed PMID: 32044314)
Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–2089. https://doi.org/10.1111/liv.14548. (Epub 20200726 PubMed PMID: 32478487)
Nguyen VH, Le MH, Cheung RC, Nguyen MH. Differential clinical characteristics and mortality outcomes in persons with NAFLD and/or MAFLD. Clin Gastroenterol Hepatol. 2021;19(10):2172–81.e6. https://doi.org/10.1016/j.cgh.2021.05.029. (Epub 20210523 PubMed PMID: 34033923)
Chan KE, Ng CH, Fu CE, Quek J, Kong G, Goh YJ, et al. The spectrum and impact of metabolic dysfunction in MAFLD: a longitudinal cohort analysis of 32,683 overweight and obese individuals. Clin Gastroenterol Hepatol. 2023;21(10):2560–9.e15. https://doi.org/10.1016/j.cgh.2022.09.028. (Epub 20221003 PubMed PMID: 36202348)
Vaz K, Clayton-Chubb D, Majeed A, Lubel J, Simmons D, Kemp W, et al. Current understanding and future perspectives on the impact of changing NAFLD to MAFLD on global epidemiology and clinical outcomes. Hepatol Int. 2023. https://doi.org/10.1007/s12072-023-10568-z. (Epub 20230809. PubMed PMID: 37556065)
Kawaguchi T, Tsutsumi T, Nakano D, Torimura T. MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol Res. 2022;52(5):422–432. https://doi.org/10.1111/hepr.13706. (Epub 20210917 PubMed PMID: 34472683)
Lim S, Kim JW, Targher G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol Metab. 2021;32(7):500–514. https://doi.org/10.1016/j.tem.2021.04.008. (Epub 20210508 PubMed PMID: 33975804)
Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936–944. https://doi.org/10.1111/jgh.13264. (PubMed PMID: 26667191)
Cariou B, Byrne CD, Loomba R, Sanyal AJ. Nonalcoholic fatty liver disease as a metabolic disease in humans: a literature review. Diabetes Obes Metab. 2021;23(5):1069–1083. https://doi.org/10.1111/dom.14322. (Epub 20210210. PubMed PMID: 33464677; PubMed Central PMCID: PMC8248154)
Huang Q, Zou X, Wen X, Zhou X, Ji L. NAFLD or MAFLD: which has closer association with all-cause and cause-specific mortality?-results from NHANES III. Front Med (Lausanne). 2021;8:693507. https://doi.org/10.3389/fmed.2021.693507. (Epub 20210701. PubMed PMID: 34277667; PubMed Central PMCID: PMC8280321)
Liang Y, Chen H, Liu Y, Hou X, Wei L, Bao Y, et al. Association of MAFLD with diabetes, chronic kidney disease, and cardiovascular disease: a 4.6-year cohort study in China. J Clin Endocrinol Metab. 2022;107(1):88–97. https://doi.org/10.1210/clinem/dgab641. (PubMed PMID: 34508601; PubMed Central PMCID: PMC8684479)
Song SJ, Lai JC-T, Wong GL-H, Wong VW-S, Yip TC-F. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2023. https://doi.org/10.1016/j.jhep.2023.07.021
Funding
This study was partially supported by Medica Sur Clinic & Foundation. JG is supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney; a National Health and Medical Research Council of Australia (NHMRC) Program Grant (APP1053206), Project, Ideas and Investigator Grants (APP2001692, APP1107178, APP1108422, APP1196492) and a Cancer Institute, NSW grant (2021/ATRG2028).
Author information
Authors and Affiliations
Contributions
Conception and design: NM-S. Material preparation, data collection and analysis were performed by MMR-M, NM-S and CJ-G. The first draft of the manuscript was written by MMR-M and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Medica Sur Clinic & Foundation (protocol code 2021-EXT-552 and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramírez-Mejía, M.M., Jiménez-Gutiérrez, C., Eslam, M. et al. Breaking new ground: MASLD vs. MAFLD—which holds the key for risk stratification?. Hepatol Int 18, 168–178 (2024). https://doi.org/10.1007/s12072-023-10620-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10620-y