Abstract
Background
We evaluated the dynamics of hepatic encephalopathy (HE) and ammonia estimation in acute-on-chronic liver failure (ACLF) patients due to a paucity of evidence.
Methods
ACLF patients recruited from the APASL-ACLF Research Consortium (AARC) were followed up till 30 days, death or transplantation, whichever earlier. Clinical details, including dynamic grades of HE and laboratory data, including ammonia levels, were serially noted.
Results
Of the 3009 ACLF patients, 1315 (43.7%) had HE at presentation; grades I–II in 981 (74.6%) and grades III–IV in 334 (25.4%) patients. The independent predictors of HE at baseline were higher age, systemic inflammatory response, elevated ammonia levels, serum protein, sepsis and MELD score (p < 0.05; each). The progressive course of HE was noted in 10.0% of patients without HE and 8.2% of patients with HE at baseline, respectively. Independent predictors of progressive course of HE were AARC score (≥ 9) and ammonia levels (≥ 85 μmol/L) (p < 0.05; each) at baseline. A final grade of HE was achieved within 7 days in 70% of patients and those with final grades III–IV had the worst survival (8.9%). Ammonia levels were a significant predictor of HE occurrence, higher HE grades and 30-day mortality (p < 0.05; each). The dynamic increase in the ammonia levels over 7 days could predict nonsurvivors and progression of HE (p < 0.05; each). Ammonia, HE grade, SIRS, bilirubin, INR, creatinine, lactate and age were the independent predictors of 30-day mortality in ACLF patients.
Conclusions
HE in ACLF is common and is associated with systemic inflammation, poor liver functions and high disease severity. Ammonia levels are associated with the presence, severity, progression of HE and mortality in ACLF patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic encephalopathy (HE) is a cardinal decompensation, which affects about one-third of patients [1] and remains the most common reason for hospitalization amongst cirrhosis patients [2]. The costs associated with HE is enormous (11.6 billion$) and outweigh other decompensating events in cirrhosis [3] or other chronic diseases [2, 4].
The presentation of cirrhosis patients with HE can vary from trivial alterations in cognition, impairment in driving skills, altered behavior to deep coma [5]. Further, the occurrence of HE is associated with high 1-year mortality in cirrhosis [6, 7]. New onset of HE may encompass acute decompensation (AD) or acute-on-chronic liver failure (ACLF) in cirrhosis patients according to the European definition (EASL), which connotes varying prognosis depending on the presence of other extrahepatic organ failures [6, 8]. In contrast, ACLF, as per the Asia Pacific definition (APASL), encompasses a homogenous population with chronic liver disease or cirrhosis (without prior decompensation) with an acute hepatic insult manifesting as jaundice and coagulopathy with ascites and or encephalopathy that is associated with high short-term mortality [9]. Like traditional decompensated cirrhosis (DC), HE in APASL-ACLF is also associated with increased mortality independent of other organ failures [10]. However, limited literature exists regarding the unique characteristics of HE in ACLF patients [6]. HE, in EASL-ACLF patients, occurs at a younger age, likely associated with alcoholism, systemic inflammatory response, liver failure and poor outcomes [10, 11]. A recent review reported five retrospective studies demonstrating the independent association of HE with mortality in APASL-ACLF patients [12]. We also developed an AARC-model with HE grade as a critical determinant of mortality in APASL-ACLF patients [13]. However, the presence of HE, its evolution over time and its association with disease severity, systemic inflammation and organ failures are poorly characterized in APASL-ACLF patients.
Ammonia, systemic inflammation, gut dysbiosis and neuronal inflammation are the key players in the pathogenesis of HE in ACLF patients [6, 11, 12]. However, conflicting evidence on ammonia levels as a predictor of HE and mortality in cirrhosis is prevalent [14]. EASL/AASLD guidelines also do not support the use of ammonia as a prognostic marker in cirrhosis [15]. On the contrary, the recent studies support the use of ammonia as a predictor of the severity of HE, organ failures and mortality in EASL-AD and EASL-ACLF patients [11, 16]. The significance of ammonia estimation in APASL-ACLF is not well characterized. Therefore, we planned this study to assess the natural history of HE and evaluate the importance of ammonia on the presence, severity, progression of HE and to understand its impact on mortality in APASL-ACLF patients.
Methods
Patients
This study analyzed prospectively collected data from the APASL-ACLF Research Consortium (AARC) database (31 centres) between April 2009 and December 2019. The data were collected online at www.aclf.in and validation were carried out at the ILBS, New Delhi, India. In the data validation, we resolved the coding errors and conflicts. The detailed data were collected about demographic, clinical and laboratory parameters starting from admission till day 30 or death, transplantation, or discharge, whichever was earlier. Informed consent and ethics approval (file number: F/25/5/64/AC2013/912) was taken at the central level and individual centres of recruitment.
Patient selection All patients ≥ 18 years of age diagnosed with ACLF according to the APASL definition and consenting to be a part of the study were included [9]. Patients who survived < 24 h, prior decompensation, acute liver failure (ALF), pregnancy, hepatocellular carcinoma, or extrahepatic malignancy were excluded (Table S1).
Assessment of hepatic encephalopathy The presence of HE was diagnosed by the expert hepatologists as an impairment of cognition, consciousness, or motor function after excluding other causes of mental disturbances [9]. The West Haven scale was used to assess the severity of HE. Patients were categorized broadly in two levels; namely, organ dysfunction: Grades I–II HE and organ failure: Grades III–IV HE [11]. The dynamicity of HE was defined as the evolution of HEs grades in ACLF patients over 4, 7, 15 and 30-days after the enrolment. The ‘Final’ grade of HE was defined as the grade of HE attained before death, transplantation, discharge, or 30 days, whichever was earlier. The course was labelled “static” when the baseline and final grade of HE was the same. “Progressive” course was defined when the grade of HE worsened from HE I–II to HE III–IV and progression from no-HE to the development of HE. “Improving” course was labelled when the grades improved from HE III–IV to HE I–II, HE III–IV to no-HE and HE I–II to no-HE.
Laboratory assessment
Laboratory investigations included a complete hemogram, serum electrolytes, renal and liver function tests, and complete coagulogram, arterial blood gas analysis with lactate level. Ammonia estimation was performed immediately using the Ammonia Checker-II (Daiichi Kagaku Co Ltd, Kyoto, Japan) using finger-prick blood. An upper gastrointestinal endoscopy was performed in all patients to detect the presence of oesophageal varices and hepatic venous pressure gradient (HVPG) was noted, if available. The severity of liver disease was determined by Child–Turcotte–Pugh (CTP), model for end-stage liver disease (MELD), MELD sodium (MELD-Na), sequential organ failure assessment (SOFA), AARC score and Acute Physiology and Chronic Health Evaluation (APACHE-II).
The treatment was given according to the APASL guidelines [9]. Briefly, rifaximin and lactulose were given for HE, nutrition, organ support, antibiotics, renal replacement, mechanical ventilation and other supportive care as needed. Patients who underwent liver transplants (n = 40) or recruited for experimental therapies were excluded.
Statistical analysis
Categorical variables were expressed as proportions (percentage) and continuous variables as mean (standard deviation) or median (interquartile range; IQR), as appropriate. Comparative analysis for categorical variables was performed with the Chi-square test or Fisher exact test. Continuous variables were compared between two groups using the t/u test for nonskewed/skewed data. Numerical data were compared between three groups on ANOVA or Kruskal–Wallis ANOVA for nonskewed and skewed data, respectively. Post hoc Bonferroni test was done for pairwise comparisons. Within-group comparisons of numerical data were made on repeated measures ANOVA, with post hoc Bonferroni test for pairwise comparisons. Multivariable logistic regression was done to assess features associated with HE at baseline. The significant predictors on univariable analysis were entered in the multivariable model with backward elimination. The model with the highest area under receiver operator curve (AUROC) was retained. Univariate analysis followed by the entry of significant parameters into a multivariable competing-risk Cox-regression model was done to evaluate independent predictors of in-hospital incident-HE and keeping death as a competing event. The predictors of death at 30 days of the presentation were analyzed on multivariable Cox regression. The final model was selected based on the best Harrell’s C-index and Somers’ D. The cumulative probability of survival was illustrated on the Kaplan–Meier graph and survival estimates were compared using the Log-rank test. AUROC, precision–recall plots, Youden’s index and F1 score were analyzed to derive ammonia cutoffs for optimal sensitivity, specificity, positive predictive and negative predictive values for 30-day mortality. A predefined sensitivity and specificity threshold at 90% each and PPV at 100% were set to identify ammonia cutoffs for classifying patients into green, yellow, red and lethal zone. All tests were two-tailed with p < 0.05 was considered significant and adjusted for multiple-groups comparisons when necessary. The missing data were deemed null during analysis. The analysis was performed using IBM-SPSS-version 26 and STATA-version 16.
Results
Baseline characteristics of the study population
The baseline characteristics of the ACLF patients, overall and with or without HE, are illustrated in Table 1. The mean age at presentation was 44.6 (12.5) years and the majority were males (n = 2549; 84.7%). Alcohol abuse was the commonest acute precipitant as well as the underlying cause of chronic liver disease. Ascites was the commonest decompensation (91.4%) with jaundice and coagulopathy in all patients. Out of 3009 patients, 1315 (43.7%) patients had HE at presentation (Figure S1). The presence of HE without ascites was uncommon (8.6%). Of patients with HE (n = 1315), Grades I-II HE were noted in 981 (74.6%) and Grades III-IV in 334 (25.4%) patients. Although the proportion of patients with ascites was equally represented in cohorts with and without HE, the severity of ascites was higher in the former than in the latter cohort (p < 0.001). The presence of SIRS, sepsis, organ failures and severity scores, such as CTP, MELD, MELD-Na, SOFA, APACHE-II and AARC scores was significantly higher in patients with-HE as compared to those without-HE (p < 0.001; each). Leukocytosis, reduced platelet counts and hemoglobin, deranged renal functions, impaired liver functions (elevated bilirubin, alanine amino-transferase and international normalized ratio; INR), hypoproteinemia, low alpha-fetoprotein, elevated lactate and high ammonia levels were more commonly encountered in patients with HE as compared to those without HE at baseline (p < 0.05; each). HVPG levels were not different in ACLF patients with and without HE (p = 0.358).
Predictors of HE at baseline
On multivariable analysis, the parameters independently associated with the presence of HE (Table 2) at baseline were age, number of systemic inflammatory response syndrome (SIRS) components, ammonia levels, serum protein, sepsis and MELD score. The discrimination ability of this model was 0.777.
Natural history of HE in patients with ACLF
The final grade of HE (data available for 1718 patients) was achieved within 7 days in most patients (1199; 70%) and within 8–14 days in 305; 18% patients and 15–30 days in 214; 13% patients (Figure S2). The final assessment was no-HE in 1404 patients (81.7%), HE I-II in 188 patients (10.9%) and HE III–IV in 126 patients (7.3%) (Figure S1).
Amongst patients without HE at baseline (n = 1065), the overall course was progressive in 114 patients (10.1%) and static in 958 patients (89.9%) (Fig. 1). Amongst patients with HE at baseline (n = 653), the overall course was progressive in 62 (8.2%), static in 135 (20.6%) and improving in 465 patients (71.2%).
The natural history of HE was also assessed as per the grade of HE at baseline. Of 1065 patients with no HE at baseline, a few patients progressed to develop HE at day 4 (n = 71; 7%), day 7 (n = 92; 9%) or at final assessment (n = 107; 10%) (Fig. 1). Of the 495 patients with organ dysfunction (grades I–II HE) at baseline, there was a resolution towards no-HE at day 4 (n = 221; 45%), at day 7 (n = 295; 60%) and a final assessment (n = 348; 70%). Progression to HE III–IV was noted among few such patients at day 4 (n = 31; 6%), day 7 (n = 55; 11%) and at final assessment (n = 53; 11%). Static grade of HE was noted in (n = 243; 49%) at day 4, (n = 145; 29%) at day 7 and (n = 94; 19%) patients at final assessment in these patients (Fig. 1). Of the 158 patients with grades III–IV HE at baseline, 56 (25%), 81 (51%), 98 (62%) patients improved to no-HE at days 4, 7 and final assessment, respectively. Forty-four (28%), 28 (18%) and 19 (12%) patients improved to grades I–II HE at days 4, 7 and final assessment amongst these patients with baseline HE III–IV. The static grade of HE III–IV was noted in 58 (37%), 49 (31%) and 41 (26%) patients at days 4, 7 and final assessment out of all patients with HE III-IV at baseline (Fig. 1).
Predictors of incident and progressive course of HE
On univariate analysis (Table S2), the patients who developed in-hospital HE were likely to have SIRS, a higher number of organ failures; especially liver, coagulation and renal, poorer severity scores, such as CTP, MELD, MELD-Na, APACHE-II and AARC score (p < 0.05; each). Low hemoglobin, leucocytosis, hyponatremia, hyperammonemia, deranged renal functions, poor liver functions (bilirubin levels, hypoproteinemia, hypoalbuminemia and elevated INR) as compared to those who did not develop HE (p < 0.05; each). The independent predictors for both incident HE and progressive course of HE (Table 2) were AARC score and ammonia levels at baseline. APASL ACLF research consortium (AARC) score ≥ 9 (sensitivity: 85%) and ammonia levels ≥ 85 μmol/L (sensitivity: 80%) could predict progressive course of HE.
Outcomes of ACLF patients according to severity and evolution of HE
Cross-sectional grade of HE
Thirty-day overall survival (Figure S3A) in patients with HE grades III–IV was the lowest compared with grades I–II HE and no-HE at baseline (31.4% vs. 51.5% vs. 78.0%; p < 0.001 overall and for each comparison). The overall survival dropped further amongst grades III–IV HE cases at day 4 (14.7%) and final assessment (8.9%). According to the cross-sectional grades of HE at day 7 and the final assessment, the overall survival as described in Figures S3B and S3C shows lower survival in grades III-IV HE when compared with grades I-II HE and no-HE (p < 0.001 overall and for each comparison).
Evolution of HE
Thirty-day overall survival, according to the evolution of HE, is illustrated in Fig. 2. Amongst patients without-HE at baseline, progression to grades III-IV HE and grades I-II HE conferred lower survival than no-progression of HE at final assessment (20.6% vs. 51.2% vs. 82.3%; p < 0.001 overall and for each comparison). Amongst patients with HE I–II at baseline, the progression to grades III-IV HE or static disease in grades I–II HE conferred a poor survival as compared to improvement to no-HE (11.3% vs. 22.1% vs. 60.2%; p < 0.001 overall and for each comparison). Amongst patients with baseline HE III–IV, the overall survival was relatively low. The patients who were static in HE grades III–IV had the worst survival (2.4%) as compared to those who improved to HE grades I-II (26.1%) or no-HE (39.7%) (p < 0.001 overall).
Role of ammonia in patients with ACLF
The ammonia levels were higher in patients with HE than those without HE at baseline (p < 0.001) (Table 1), which were further higher in patients with grades III-IV HE (median: 193; IQR: 103–284) as compared to patients in grades I-II HE (median: 131; IQR: 87–179) and in no-HE (median: 102; IQR: 66–141) (p < 0.001 overall and for each comparison) (Figure S4). Serial trends of serum ammonia over 7 days were also significantly different between different grades of HE (p < 0.001) (Figure S5). Amongst patients without HE at baseline, there was a trend towards an increase in ammonia levels in those who progressed and developed HE [+ 53.7% (IQR: 9.9–97.5)] than in those who remained without HE [+ 15.5% (IQR: − 1.8 to 32.8)]; p = 0.074 (Figure S6A). Amongst patients with HE at baseline, there was an increase in ammonia levels in those who progressed to higher grades of HE [+ 3745% (IQR: − 49.5 to + 12,490.0)] than in those who were static [+ 245.0% (− 217.0 to 708.0)] or had an improvement in HE grades [+ 10.3 (− 9.9 to 30.4)]; p = 0.005 (Figure S6B).
On ROC analysis, the ammonia levels at days 0, 4 and 7 were significant predictors of 30-day mortality in ACLF (p < 0.001 for each) (Fig. 3). In comparison, the ammonia levels at day 4 had the best discrimination (AUROC: 0.848; 95% CI 0.792–0.904; p < 0.001) than at day 0 or day 7 (p = 0.048 for day 0 and day 4 comparison and not significant for day 0 and day 7 or day 4 and day 7 comparisons).
Role of ammonia evaluation in acute on chronic liver failure patients. a receiver-operating curve for 30-day mortality with ammonia levels at days 0, 4, 7 (D0, D4, D7); AUC: Area under ROC curve. b Precision-Recall curve for 30-day mortality with ammonia levels at days 0, 4, 7 (D0, D4, D7). c Ammonia cut-offs for at predefined sensitivity, specificity and positive predictive values for predicting 30-day mortality. d Ammonia cutoffs for 30-day mortality with respective sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and F1 score
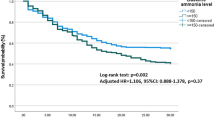
The ammonia cutoffs of 85, 170 and 240 μmol/L on day 4 of the presentation could classify patients in the green zone, red zone and lethal zone. Patients in the green, red and lethal zone had a 30-day survival of about 80%, 20% and 0%, respectively. The cutoffs for 30-day mortality balanced as per Youden’s index and F1 score were 109, 99 and 130 μmol/L for day 0, day 4 and day 7 ammonia levels values. The 30-day survival in a cohort with day 4 ammonia > 170 μmol/L was 24.6% than with ammonia < 170 μmol/L 62.5%; p < 0.001 (Figure S7). The ammonia cutoffs for predicting 7-day mortality were 50 (sensitivity: 90%), 216 (specificity: 90%) and 190 μmol/L (optimal Youden’s index). The likewise cutoffs for predicting 14-day mortality were 60 (sensitivity: 90%), 210 (specificity: 90%) and 160 μmol/L (optimal Youden’s index).
Serial trends of ammonia at days 0, 4 and 7 of the presentation separated 30-day survivors from nonsurvivors (p < 0.001) (Fig. 4) in ACLF patients. There was a + 61.0% (IQR: + 16.8 to + 143.8) increase in ammonia levels amongst 30-day nonsurvivors than − 30% (IQR: − 63.6 to + 21.0) decrease amongst survivors of ACLF (p < 0.001).
Predictors of short-term mortality in patients with ACLF
Overall, 30-day survival in the whole cohort (n = 1718), with-HE and without-HE, was 48.7% and 80.1%; p < 0.001. On multivariable cox-regression (Table S3), the independent predictors at baseline for 30-day mortality were the presence of HE (HR: 1.894), SIRS (HR: 1.663), INR (HR: 1.307), creatinine (HR: 1.194), lactate (HR: 1.141), bilirubin (HR: 1.033), age (HR: 1.023) and ammonia (HR: 1.002). The Harrell’s C (0.752) and Somers’ D (0.504) of this model were the highest amongst multiple other models tested with well-known predictors of mortality, such as MELD, CTP and CLIF-SOFA.
The results were broadly consistent across various aetiologies of ACLF (Table S3).
Discussion
This study describes the clinical characteristics of HE in a large population of APASL-ACLF patients. HE was noted at a presentation in about half of patients, which amounted to organ dysfunction in 3/4th and organ failure in 1/4th of patients. The SIRS, hyperammonemia, hypoproteinemia, sepsis, and high MELD could distinguish ACLF patients with HE from those without HE. This signifies an important role of systemic inflammation, infections, hyperammonemia and impaired liver functions in the pathogenesis of HE in ACLF patients [12]. The patients with HE in our study had a higher number of organ failures, SOFA and APACHE-II scores, conferring profound sickness in these patients. Lower AFP and protein levels in patients with HE would represent poor liver regeneration in ACLF patients. Similarly, Cordoba et al. showed that ACLF patients with HE had leukocytosis and worse liver and renal functions than those without ACLF [17]. Recently, Shalimar et al. [16], had shown leukocyte count, coagulation and respiratory failure as the predictors of HE in AD of cirrhosis. On multivariate analysis, ammonia, INR and creatinine were the independent predictors of higher HE grades, concordant with our study [16].
We described HEs natural history, which was dynamic. Most patients (70%) achieved their final grade within 7 days of presentation, suggesting a need for an observational period before allocating definite treatment in such patients. The progressive course was noted in 10% of patients and high severity scores (AARC) and elevated ammonia levels were independently associated with the new onset and progression of HE. This would justify a need for meticulous evaluation and targeted prophylaxis of HE in patients with high severity and elevated ammonia levels. The severity of HE either during the evolution of disease portended poor prognosis on the survival of ACLF patients. Worst outcomes were remarkable in patients with a progressive course or HE grades III–IV either at baseline or during the final assessment. Likewise, Cordoba et al. reported higher mortality in ACLF patients with HE [17]. Sawhney et al. [11] showed that ACLF patients with HE had higher mortality (66% vs. 33%). They also showed that the mortality increased with higher HE grades (grade 0–1: 33%; grade 2: 59%; and grade 3–4: 76%), similar to our study. However, to the best of our knowledge, HEs dynamic evolution and its association with survival in APASL-ACLF patients were described for the first time in our study. Further, these findings emphasize a need for early control of HE in APSL-ACLF patients to achieve better outcomes.
We also dissected the significance of ammonia in ACLF patients. Ammonia levels were independently associated with the presence, grade and progression of HE, disease severity and mortality in ACLF patients. Also, day-4 ammonia levels > 170 were associated with poor survival (25%). In literature, elevated ammonia levels have been associated with HE in ACLF patients [11]. Shalimar et al. [16] showed that the ammonia levels of ≥ 79.5 μmol/L were associated with a higher incidence of HE (46.1 vs. 33.6%) and 28-day mortality in AD patients. Sawhney et al. [11] had shown a failure of reduction or increase in ammonia over the first 24 h to be associated with mortality in ACLF patients. Elevated ammonia was also reported in two small studies to predict in-hospital mortality in decompensated cirrhosis [12]. Shalimar et al. also showed an increase in ammonia levels from baseline to day 5 was associated with an increased risk of mortality and with the progression of HE in AD patients [16]. We demonstrated that ammonia > / = 88.5 μmol/L and AARC > / = 9 were the predictors of HE progression, which may be used at primary/secondary care level to stratify patients into a high-risk category with a need for referral to transplant-available centers. Further, we showed an increase in ammonia over 4–7 days by 60% or cross-sectional assessment at day 4 > 170 μmol/L to predict mortality in ACLF patients. This would guide physicians in making appropriate and timely decisions for liver transplantation or bridging therapies. Also, a reduction in ammonia by 30% reflected survival in our study, which supports the idea of ammonia as a therapeutic target amongst ACLF patients. Further, this is supported by a Cochrane review, which concluded l-ornithine l-aspartate administration, which reduces ammonia levels associated with a reduction in mortality in cirrhosis patients [18].
Finally, given the observations, we hypothesize that HE in ACLF should be categorized as a separate entity. HEs phenotype in ACLF behaves like a combination of features observed in ALF and DC. Based on our previous observations [19] and the current study, we demonstrated that HEs presence and progression in ACLF were associated with systemic inflammation, which is quite similar to ALF patients [20]. We showed that hyperammonemia was associated with the presence, grade and progression of HE in ACLF patients, identical to ALF [21], but not the DC patients [22]. Ammonia levels were associated with mortality in ACLF patients, which has been reported in ALF patients [21], but not in DC patients [22]. The presence of cerebral edema was previously shown in ACLF patients with HE, which worsened with increasing HE and systemic inflammation [19], which paralleled the features seen in ALF patients [6]. Association of HE with cirrhosis, poor liver synthetic functions and portosystemic shunting in ACLF patients with HE would represent similarity with DC patients. We showed comparable HVPG levels in ACLF patients with and without HE, which would mean extensive portosystemic collateralization in the former group. The pathophysiology and the mortality in ACLF patients with HE are likely to follow a middle path between type A and type B/C HE. Plasmapheresis and liver dialysis can improve HE in ALF and ACLF patients [23], representing common pathobiology in both groups of patients. Liver transplantation is also deemed urgent in ACLF patients with HE as in ALF patients. Therefore, we propose that HE in ACLF be coined as type D HE for uniformity, research and prognostic reasons. Further studies are needed to validate this hypothesis.
Strengths of the study include a large number of patients, multicentric collaboration, comprehensive description and analysis of the natural history of HE and outcomes in ACLF. Limitations include the impact of renal dysfunction and its relation with ammonia levels were not studied. The data on acute precipitants and treatment given for HE were not available. Findings are generalizable to ACLF patients by the APASL definition. Technical problems and methodological issues with ammonia estimation were possible across centres, although investigators ensured the reliability of estimation before data entry. Sarcopenia and frailty are increasingly recognized and found negatively associated with survival in ACLF patients. Such data were not available in the current study. Further, comparative analysis in patients with ACLF who have previous decompensations is needed to develop a more holistic approach towards HE in ACLF.
In conclusion, HE in APASL-ACLF is a common decompensation, which progresses in about 10% of patients. HE is independently associated with systemic inflammation, multi-organ failures, poor liver functions and high mortality. Serial evaluation of ammonia and HE grades can predict outcomes in ACLF patients.
Abbreviations
- HE:
-
Hepatic encephalopathy
- ACLF:
-
Acute-on-chronic liver failure
- EASL:
-
European Association for the Study of the Liver
- APASL:
-
Asian Pacific Association for the Study of Liver
- DC:
-
Decompensated cirrhosis
- ALF:
-
Acute liver failure
- ICU:
-
Intensive Care Unit
- AARC:
-
APASL ACLF Research Consortium
- HVPG:
-
Hepatic venous pressure gradient
- CTP:
-
Child-Turcotte-Pugh score
- MELD:
-
Model for endstage liver disease
- MELD-Na:
-
MELD-sodium
- SOFA:
-
Sequential Organ Failure Assessment score
- APACHE-II:
-
Acute Physiology and Chronic Health Evaluation-II
- IQR:
-
Interquartile range
- AUROC:
-
Area under receiver operating curve
- SIRS:
-
Systemic inflammatory response syndrome
- SHR:
-
Sub-distribution hazard ratio
- CI:
-
Confidence interval
References
Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy–definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology (Baltimore, MD) 2002;35:716–721
Hirode G, Vittinghoff E, Wong RJ. Increasing burden of hepatic encephalopathy among hospitalized adults: an analysis of the 2010–2014 National Inpatient Sample. Dig Dis Sci 2019;64:1448–1457
Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A quality improvement initiative reduces 30-day rate of readmission for patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14:753–759
Di Pascoli M, Ceranto E, De Nardi P, Donato D, Gatta A, Angeli P, et al. Hospitalizations due to cirrhosis: clinical aspects in a large cohort of italian patients and cost analysis report. Dig Dis 2017;35:433–438
Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol 2015;62:437–447
Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, et al. Hepatic encephalopathy: novel insights into classification, pathophysiology and therapy. J Hepatol 2020;73:1526–1547
Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol 2012;10:1034.e1031–1041.e1031
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144(1426–1437):1437.e1421–1429.e1421
Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int 2019;13:353–390
Cordoba J, Ventura-Cots M, Simón-Talero M, Amorós À, Pavesi M, Vilstrup H, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol 2014;60:275–281
Sawhney R, Holland-Fischer P, Rosselli M, Mookerjee RP, Agarwal B, Jalan R. Role of ammonia, inflammation, and cerebral oxygenation in brain dysfunction of acute-on-chronic liver failure patients. Liver Transpl 2016;22:732–742
Lee G-H. Hepatic encephalopathy in acute-on-chronic liver failure. Hepatol Int 2015;9:520–526
Choudhury A, Jindal A, Maiwall R, Sharma MK, Sharma BC, Pamecha V, et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol Int 2017;11:461–471
Nicolao F, Efrati C, Masini A, Merli M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with and without hepatic encephalopathy. J Hepatol 2003;38:441–446
Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology (Baltimore, MD) 2014;60:715–735
Shalimar, Sheikh MF, Mookerjee RP, Agarwal B, Acharya SK, Jalan R. Prognostic role of ammonia in patients with cirrhosis. Hepatology 2019;70(3):982–94. https://doi.org/10.1002/hep.30534
Cordoba J, Ventura-Cots M, Simon-Talero M, Amoros A, Pavesi M, Vilstrup H, et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol 2014;60:275–281
Goh ET, Stokes CS, Sidhu SS, Vilstrup H, Gluud LL, Morgan MY. l-Ornithine l-aspartate for prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev 2018;5:Cd012410
Gupta T, Dhiman RK, Ahuja CK, Agrawal S, Chopra M, Kalra N, et al. Characterization of cerebral edema in acute-on-chronic liver failure. J Clin Exp Hepatol 2017;7:190–197
Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol 2015;5:S7–S20
Bhatia V, Singh R, Acharya SK. Predictive value of arterial ammonia for complications and outcome in acute liver failure. Gut 2006;55:98–104
Haj M, Rockey DC. Ammonia levels do not guide clinical management of patients with hepatic encephalopathy caused by cirrhosis. Am J Gastroenterol 2020;115:723–728
Tan EX, Wang MX, Pang J, Lee GH. Plasma exchange in patients with acute and acute-on-chronic liver failure: a systematic review. World J Gastroenterol 2020;26:219–245
Funding
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Institutional and National) and with the Declaration of Helsinki 1975, as revised in 2008. The AARC registry for ACLF was approved by the Institutional Ethical Review Board at the nodal center, i.e., ILBS New Delhi (vide letter no F/25/5/64/AC2013/912) and all the participating centres also had necessary approval from the respective ethical board.
Informed consent
Informed consent was obtained from all individual participants or legally acceptable representatives of the participant included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, N., Dhiman, R.K., Choudhury, A. et al. Dynamic assessments of hepatic encephalopathy and ammonia levels predict mortality in acute-on-chronic liver failure. Hepatol Int 15, 970–982 (2021). https://doi.org/10.1007/s12072-021-10221-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10221-7