Abstract
Background
Various antibiotic regimens are used for primary and secondary prevention of spontaneous bacterial peritonitis (SBP). A systematic review and network meta-analysis to compare various antibiotics regimens for primary and secondary prevention of SBP were done.
Methods
We did a comprehensive literature search using various databases (i.e. MEDLINE via Ovid and PubMed, Embase, Cochrane Central Register of Controlled Trials and others) from inception to 26th October 2019 using various keywords. Only randomised studies which evaluated the role of antibiotics in adult cirrhotic patients with ascites for primary or secondary prophylaxis of SBP were included. The primary outcome was occurrence/recurrence of SBP episode and other outcomes assessed were extra-peritoneal infections and reduction in mortality. We did random-effects network meta-analysis using a Bayesian approach, and calculated odds ratios (ORs) and 95% credible intervals (CrI); agents were ranked using rank probabilities.
Results
We found total 1701 records in our systematic database search and out of these 17 randomised trials were found eligible for network meta-analysis. For primary prevention of SBP, the odds ratio (95% CrI) for norfloxacin daily was 0.061 (0.0060, 0.33) and for rifaximin daily was 0.037 (0.00085, 0.87) and norfloxacin and rifaximin alternate month was 0.027 (0.00061, 0.61) when compared to placebo or no comparator. For the secondary prevention of SBP, rifaximin daily had odds of 0.022 (0.00011, 0.73).
Conclusion
Rifaximin is useful for both primary and secondary prevention of SBP whereas norfloxacin daily and alternate norfloxacin and rifaximin are useful for primary prophylaxis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous bacterial peritonitis (SBP) is an important infective complication of cirrhosis which may occur in 7–30% of hospitalised patients and 1.5–3.5% of outpatients [1, 2]. The occurrence of SBP is a marker of advanced liver disease [3]. The low-protein ascites in patients with end-stage liver disease have low opsonic activity and is prone to get infected [3, 4]. SBP is usually due to Gram-negative mono-bacterial infection of ascitic fluid. SBP is believed to result from increased bacterial translocation of gut microbes [1]. Intestinal dysmotility, the occurrence of small intestinal bacterial overgrowth, generalised immune dysfunction, and low opsonic activity in cirrhotic ascites contribute to the risk of SBP [5, 6]. The intestinal origin of SBP is suggested by the spectrum of organisms usually implicated i.e. Gram-negative bacilli although recent trends indicate a shift in the pattern of causative organisms [2, 7]. Although the outcomes in patients with SBP have improved, it is still associated with substantial mortality especially if the causative organisms are drug-resistant [8].
The patients with a particularly low protein in ascitic fluid (< 1.5 g/dL), advanced liver disease and deranged renal function (creatinine of > 1.2 mg/dL) have heightened risk of developing SBP and are candidates for primary prophylaxis [9, 10]. Also, those with a previous episode of SBP are at an increased risk of recurrence of SBP and usually receive secondary prophylaxis. Various antibiotics in different regimens (daily administration, alternate day therapy and weekly) have been used for these indications [10–26]. It is unclear if any particular antibiotic regimen is associated with better outcomes vis-a-vis the other regimens. Therefore, we performed a systematic review and network meta-analysis of randomised trials comparing the use of various antibiotic regimens with each other or with controls to determine the most appropriate antibiotic regimen for primary and secondary prophylaxis of SBP.
Methodology
The study was conducted and reported according to the PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions.
Search strategy and study selection
We did a comprehensive literature search in various databases (i.e. MEDLINE via Ovid and PubMed, Embase, Scopus, Web of science, LILAC, CENTRAL (Cochrane Central Register of Controlled Trials) and clinical trials.gov from inception to 26th October 2019. The detailed search strategy is shown in Supplementary Table 1. Two study reviewers (HS and BLB) did a separate comprehensive electronic search without any restrictions. The search used the following keywords “liver cirrhosis”, “chronic liver disease”, antibiotic, norfloxacin, rifaximin, ciprofloxacin, cotrimoxazole, “primary prevention” and “secondary prevention”. The bibliography of all relevant studies was manually reviewed to identify relevant studies. Two reviewers (HS and DM) independently reviewed each study for inclusion criteria and excluded non-relevant studies with discrepancies being discussed and resolved with other reviewers (VS and BLB).
Eligibility criteria
Randomised control studies were included if they met the following inclusion criteria: studies evaluating antibiotic as prophylactic intervention either for SBP, other bacterial infections or mortality in adult patients of liver cirrhosis with ascites, with (secondary prophylaxis) or without (primary prophylaxis) history of previous SBP episodes. The “placebo” group included the patients who received placebo as well as those who did not receive any intervention (controls). The antibiotic regimens were further grouped on the basis of the number of days of intervention/dosing in a week, into daily or intermittent. Daily administration for the purpose of this meta-analysis included those groups where the administration was for ≥ 5 days in a week. Intermittent administration included administration at frequency < 5 days in a week and included an alternate day or once a week administration. The combination of two antibiotics in a regimen was grouped as a separate intervention group for evaluation. We included only those studies where a minimum duration of therapy of at least 4 weeks was administered. We excluded studies in which study population had compensated liver cirrhosis, studies where antibiotics were administered only for a period of hospitalisation (for indications like gastrointestinal bleeding or treatment of SBP) or for other indications (e.g., hepatic encephalopathy). We also excluded non-randomised studies e.g. observational studies, case series, studies without a relevant comparator, reviews and studies that were published in non-English language. However, systematic reviews, meta-analysis and other important studies were reviewed to identify potentially eligible trials.
Study groups and outcomes
Included studies were categorised on the basis of use of antibiotics either for the primary (prevention of the first episode of SBP in patients without a previous episode of SBP) or secondary prophylaxis (prevention of recurrence of SBP in patients with a prior episode of SBP). For the mixed studies which reported both the subsets, we made an effort to extract data separately for the primary and secondary prophylaxis. In case, this extraction was not feasible we included such studies (mixed studies) in the aggregate analysis where all studies irrespective of nature of prophylaxis were combined.
The outcomes assessed in each category include occurrence/recurrence of SBP, the occurrence of any other bacterial infection (extra-peritoneal infections) and mortality. The definition of SBP episode (both occurrence/recurrence) was polymorphonuclear cell count in ascitic fluid equal to or higher than 250/mm3 in the absence of intra-abdominal causes of infection [25] and also included other variant such as culture-negative neutrocytic ascites (CNNA) [16]. Microbiological details of infections which occurred with the use of various antibiotic regimens were also analysed.
Data extraction
Data extraction was done with a predefined form to capture data on study characteristics, participants-related, interventions-related and bacteriological data. Reviewers (HS, BLB and SM) independently reviewed and extracted data from included studies and discrepancies were resolved by consensus.
The geometry of the network
The network plot was constructed for each outcome in all three categories (primary, secondary, aggregate) to assess the geometry of the treatment network. In all the network plots, the circle represents the intervention and thickness of the line connecting the circle are proportional to the available number of studies. The network plot was assessed for a closed triangle for the conduct of node-splitting analysis which enables comparison between the direct effects (available information) with that of indirect effect obtained by network meta-analysis.
Risk of bias assessment
Potential biases related to individual studies on selected study outcome (SBP) were assessed for evidence of bias with the Cochrane revised tool to assess the risk of bias in randomized trials (RoB 2.0 tool). Risk of bias was assessed in duplicates by two authors independently (HS and BLB), with disagreements addressed by re-evaluation, in conjunction with a third reviewer (VS) as per the RoB 2.0 tool.
Statistical analysis
For all the outcomes, the data from the intention-to-treat analysis were extracted and were dichotomous in nature. The odds ratio along with 95% CrI (Credible Interval) for each outcome was calculated by Bayesian network meta-analysis methodology. For the rank probability, the SBP occurrence/recurrence in each of the three categories was considered and the ranks of the intervention in each category described.
Methodology of analysis
We performed an arm based network meta-analysis using a Bayesian approach. Individual rows consisted of treatment arm and included data from all the included studies. R statistical software was used for statistical analysis (version 3.5.2; R Foundation, Vienna, Austria) and ‘gemtc’ along with its dependency packages were used. A logit likelihood function was used for the construction of a Bayesian random-effects network model (consistency model). The uniform standard deviation was assumed for heterogeneity prior and the initial values for modelling were heuristically imputed based on the outcome scale. The convergence diagnostics namely point scale reduction factor (Gelman Rubin diagnostics) and Gelman Rubin plot were used for modulating the burnin, inference, thinning and number of chains until it is adequate for the development of the final model. The details regarding the assessment of consistency and meta-regression have been provided in the supplementary appendix. The usefulness of random effects Bayesian network meta-analysis was shown using a directed acyclic graph (Supplementary Figure 6). Directed acyclic graph is a probabilistic graphical model that represents a set of variables and their conditional dependencies and hence would help the reader in understanding the entire metanalytic process.
Results
Search results and study characteristics
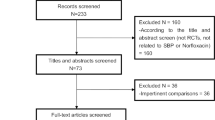
In the systematic search, 1701 citations were identified. After duplicate removal, 1135 citations were assessed for the title, abstract or full text for potentially eligible studies. Full text of 38 articles were read, of which 23 randomised studies were identified. These randomised studies were discussed for eligibility among four authors and finally 17 randomized studies were found eligible (Table 1 and Supplementary Table 2) and rest six studies were excluded for various reasons (Supplementary Table 3). The schematic diagram of the study selection (PRISMA flow chart) is shown in Fig. 1. The eligible studies were done during the period from 1990 to 2018 and the important study characteristics of each included study have been summarized in Table 1. The detailed primary objectives, inclusion–exclusion criteria and definition used have been summarized in Supplementary Table 2. We excluded certain studies for various reasons including the studies which provided treatment during hospitalization [27, 28]; when the comparison included use of additional agent other than antibiotics in one of group [29]; when antibiotics were used for prevention of hepatic encephalopathy [30, 31] and when relevant endpoints were not addressed in the study [32].
PRISMA flowchart for the selection of studies for the meta-analysis. This included four steps comprising of identification of relevant titles from various databases (Step 1A) and through manual searches (Step 1B); exclusion of duplicates (step 2A), screening of title (step 2B) or abstract of the article (step 2C). Following this relevant full-text articles were assessed for eligibility (Step 3) and eventually 17 papers fulfilling the inclusion criteria were included in the quantitative analysis (Step 4)
Summary of network geometry
The network plot of all three outcomes (occurrence/recurrence of SBP, other infections and mortality) for primary and secondary prophylaxis is depicted in Fig. 2.
Network plot of included studies for outcome category of SBP occurrence/recurrence, other infections and mortality in the category of primary and secondary prophylaxis. Each node is representing individual treatment available for comparison and the thickness between the connections according to the number of studies directly comparing the treatments
Primary prophylaxis for SBP
Total ten studies with 973 patients were included. Three studies compared ciprofloxacin with placebo [14, 15, 18], three studies compared norfloxacin with placebo [10, 13, 16], two studies compared norfloxacin with TMP-SMX [19, 23], one study each compared norfloxacin with ciprofloxacin [12] and rifaximin [11].
Secondary prophylaxis for SBP
Total nine studies with 737 patients were included. Two studies compared rifaximin with norfloxacin [25, 26], two studies compared norfloxacin with TMP-SMX [19, 23], one study each compared norfloxacin with placebo [24] and with rufloxacin [21], and one study compared ciprofloxacin with placebo [18]. Zayed et al. compared six antibiotic regimes with 30 patients in each group namely ciprofloxacin once weekly and twice-weekly, norfloxacin daily and weekly and TMP-SMX 5 days a week and once weekly [22]. One study compared norfloxacin daily and weekly ciprofloxacin [12].
The odds ratio of treatment regimens
For the outcome of SBP occurrence in the category of Primary SBP, Norfloxacin daily, Rifaximin daily and, Rifaximin Norfloxacin daily had a significant odds of 0.061 (95% CrI − 0.0060,0.33), 0.037 (0.00085, 0.87) and 0.027 (0.00061, 0.61), respectively, as compared to that of Placebo or no comparator (Fig. 3). For the outcome of SBP recurrence in the category of Secondary SBP, only Rifaximin daily had a significant difference of 0.022 (0.00011, 0.73) as compared to that of Placebo no comparator (Fig. 3). All other treatments for the outcome of SBP occurrence/recurrence in all three categories had an odds whose 95% CrI crossed unity as compared to that of Placebo or no comparator. For the outcome of other infections in all three categories namely primary, secondary and aggregate out of the 3, 3 and 5 treatments for which comparison was available against the placebo or no comparator, none were significant (Fig. 4). For the outcome of mortality in primary and secondary category out of the four and seven treatments for which comparisons were available against the placebo or no comparator, none were significant. Table 2 shows the comparison of the odds ratio of all the available treatments against each other for all three outcomes.
Rank probability of treatment regimens
In primary SBP for the outcome of SBP occurrence, the highest probability of the first rank was Rifaximin-Norfloxacin daily (0.48) followed by Rifaximin daily (0.25). In the same outcome category, for the second rank the highest probability was with Rifaximin daily (0.37) followed by Rifaximin-Norfloxacin daily (0.27) and for third rank Norfloxacin daily (0.42) followed by Rifaximin daily (0.18), respectively. The placebo or no comparator has the highest probability (0.52) of being the last rank (rank 7) (Fig. 4).
In secondary SBP for the outcome of SBP reoccurrence, the highest probability of the first rank was Rifaximin daily (0.76) followed by Ciprofloxacin intermittent (0.08). In the same outcome category, for the second rank the highest probability was with Ciprofloxacin intermittent (0.29) followed by Norfloxacin intermittent (0.16) and for third rank Ciprofloxacin intermittent (0.22) followed by equal probability among Norfloxacin daily.(0.18) and TMP/SMX daily (0.18). The placebo or no comparator has the highest probability (0.5) of being the last rank (rank 8) (Fig. 4). We have provided the model diagnostic of the final model for each outcome in all three categories as Supplementary Table 4.
The aggregate prophylaxis (including all studies for primary and/or secondary SBP) was done and the details are provided in supplementary appendix (Supplementary Table 5, Supplementary Fig. 1, 2 and 3).
The analysis of the microbiological findings of the SBP (Supplementary Table 6 and Supplementary Fig. 4), meta-regression (Supplementary Table 7) taking duration of therapy as a covariate and statistical exploration of inconsistency (Supplementary Fig. 5) have been provided in supplementary appendix. A directed acyclic graph depicting the advantage of Bayesian random effect meta-analysis has been provided as Supplementary Fig. 6.
Risk of bias across studies
The risk of bias summary about each domain of risk of bias is presented in Supplementary Table 8. Nine studies were found well-conducted and evaluated as low risk of bias in all the domains [10, 11, 13–15, 19, 20, 24, 25], two studies had ‘some concerns’ in the randomization process and overall fell in the ‘some concern’ category [16, 18], six studies were found as high risk of bias in the randomization process and counted in ‘high risk’ category as overall bias [12, 17, 21–23, 26].
Discussion
The results from our network meta-analysis suggest that use of norfloxacin or rifaximin daily were more effective than controls for primary prophylaxis of SBP. Prophylactic antibiotics for SBP are used for selective gut decontamination that could reduce Gram-negative organisms in the gastrointestinal tract and limit their translocation [16]. Two previous systematic reviews have addressed the issue of antibiotic prophylaxis for the prevention of SBP [33, 34]. One systematic review addressed only the primary prophylaxis and suggested that norfloxacin was the antibiotic of choice for primary prophylaxis. However, this review also included studies which were not directly aimed to address the issue of prevention of SBP and had studied the use of antibiotics for cirrhosis related hepatic encephalopathy (Supplementary Table 3). Also, the review did not discriminate the regimens on the basis of dosing strategy and treated all antibiotics regimens using one antibiotic as a single group. This may be fallacious as daily and weekly dosing strategy may not act alike and the efficacy and side effects of these are expected to be different [33]. Norfloxacin and rifaximin have important differences in their action and adverse effects. While fluoroquinolones are an attractive option due to their action against Gram-negative bacteria and low systemic absorption (especially norfloxacin), recent reports suggest increase in drug-resistant infections as a cause of SBP [35]. Use of fluoroquinolones (FQ) is associated with the risk of development of FQ resistant strains. Indeed, some studies demonstrate the appearance of FQ resistant strains in faeces with FQ administration [11]. Rifaximin, although costlier, has the benefit of minimal systemic bioavailability and risk of resistance is deemed to be lower [36]. Our results suggest that while daily norfloxacin and rifaximin could be acceptable options for primary prophylaxis of SBP, the use of ciprofloxacin (daily or intermittent) or trimethoprim-sulfamethoxazole is not more effective than controls. These results with regard to rifaximin should be interpreted with caution as there was only one eligible study for use as primary prophylaxis.
None of the systematic reviews previously have addressed the issue of comparison of antibiotics for the secondary prophylaxis of SBP although one meta-analysis included all the trials dealing with primary and secondary prophylaxis. Also, the authors compared one antibiotic to the other rather than antibiotic regimens [34]. Patients who are candidates for secondary prophylaxis may have a greater likelihood of underlying drug resistance related to the prior exposure to antibiotics and hospitalisation. Our results suggest that rifaximin is the only antibiotic regimen which had significant efficacy for secondary prevention. None of the regimens utilising fluoroquinolones or trimethoprim-sulfamethoxazole demonstrated significant efficacy when compared to controls. This suggests that rifaximin could be a preferred drug for secondary prophylaxis. This may be related to the fact that these patients with the possibility of prior exposure to antibiotics might harbor a greater risk of FQ resistant organisms. The choice of antibiotic for prophylaxis should also account for the risk of the emergence of multidrug-resistant strains with the use of prophylactic antibiotics. This concern is especially important with the use of FQs for prophylaxis as the majority of quinolone-resistant strains are also resistant to trimethoprim-sulfamethoxazole [35]. The European association for the study of liver (EASL) in 2018 suggested the use of norfloxacin in high-risk cirrhotic patients (low protein ascites) for primary and secondary prophylaxis of SBP. Although the guidelines did not suggest the use of rifaximin in spite of the “promising evidence’ [37]. It is unclear why the guidelines committee did not recommend the use of rifaximin but the possible reason could be small number of studies (especially the direct trials against daily norfloxacin) and higher costs. However, our meta-analysis suggests that this recommendation should be re-evaluated and future studies should focus on comparing daily norfloxacin and rifaximin. This is especially relevant in the wake of issues associated with fluoroquinolone use i.e. baseline resistance to fluoroquinolones, higher risk of acquiring methicillin-resistant Staphylococcus aureus and concerns regarding adverse effects like peripheral neuropathy and tendonitis [38].
Our analysis of microbiological data from the SBP infections demonstrates, as expected, a reduction in the risk of Gram-negative organism related SBP with most of the antibiotic regimens. Unexpectedly, ciprofloxacin daily was seen to be associated with increased risk of Gram-negative SBP. The reason is unclear but possibly related to the increased number of Gram-negative infections noted in one study. In fact, this is one of the few studies which showed a lack of efficacy of antibiotics for primary prophylaxis of SBP [14].
Our network meta-analysis has few limitations: some of the arms have been tested only in a limited number of trials or patients and we could not estimate the effect of underlying liver disease on the efficacy of prophylactic strategy because of lack of such information in the included trials. However, the separate analysis of primary and secondary prophylaxis, and separation of intermittent and daily regimens are important strengths because these results could be helpful to the clinicians to tailor the choice of antibiotics for different clinical situations. Also, we conducted meta-regression evaluating the duration of treatment as a covariate and our results suggest that duration of treatment does not seem to have impact on the results of the meta-analysis. To account for some of the large studies which did not provide data separately for primary and secondary prophylaxis we have also done an aggregate analysis and the efficacy of rifaximin did not seem to change with this analysis.
In conclusion, rifaximin seems to be efficacious in both the setting of primary as well as secondary prophylaxis whereas daily norfloxacin seems to be beneficial in the primary prevention of SBP. However, further studies should address the use of rifaximin as primary prophylaxis as the current evidence for its use is based on only a single study.
References
Ekpanyapong S, Reddy KR. Infections in cirrhosis. Curr Treat Options Gastroenterol 2019;17(2):254–270
Marciano S, Díaz JM, Dirchwolf M, Gadano A. Spontaneous bacterial peritonitis in patients with cirrhosis: incidence, outcomes, and treatment strategies. Hepatic Med Evid Res 2019;11:13–122
Schwabl P, Bucsics T, Soucek K, Mandorfer M, Bota S, Blacky A, et al. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int 2015;35(9):2121–2128
Andreu M, Sola R, Sitges-Serra A, Alia C, Gallen M, Vila MC, et al. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology 1993;104(4):1133–1138
Bauer TM, Schwacha H, Steinbrückner B, Brinkmann FE, Ditzen AK, Aponte JJ, et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol 2002;97(9):2364–2370
Runyon BA. Patients with deficient ascitic fluid opsonic activity are predisposed to spontaneous bacterial peritonitis. Hepatol Baltim Md 1988;8:632–635
Rostkowska KA, Szymanek-Pasternak A, Simon KA. Spontaneous bacterial peritonitis—therapeutic challenges in the era of increasing drug resistance of bacteria. Clin Exp Hepatol 2018;4(4):224–231
Maraolo AE, Gentile I, Pinchera B, Nappa S, Borgia G. Current and emerging pharmacotherapy for the treatment of bacterial peritonitis. Expert Opin Pharmacother 2018;19(12):1317–1325
Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. Bacterial infections in cirrhosis: a position statement based on the EASL special conference 2013. J Hepatol 2014;60:1310–1324
Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 2007;133(3):818–824
Assem M, Elsabaawy M, Abdelrashed M, Elemam S, Khodeer S, Hamed W, et al. Efficacy and safety of alternating norfloxacin and rifaximin as primary prophylaxis for spontaneous bacterial peritonitis in cirrhotic ascites: a prospective randomized open-label comparative multicenter study. Hepatol Int 2016;10(2):377–385
Yim HJ, Suh SJ, Jung YK, Yim SY, Seo YS, Lee YR, et al. Daily norfloxacin vs. weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: a randomized controlled trial. Am J Gastroenterol 2018;113(8):1167–1176
Novella M, Solà R, Soriano G, Andreu M, Gana J, Ortiz J, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology 1997;25(3):532–536
Téllez-Ávila F, Sifuentes-Osornio J, Barbero-Becerra V, Franco-Guzmán A, Ruiz-Cordero R, Alfaro-Lara R, et al. Primary prophylaxis with ciprofloxacin in cirrhotic patients with ascites: a randomized, double blind study. Ann Hepatol 2014;13(1):65–74
Terg R, Fassio E, Guevara M, Cartier M, Longo C, Lucero R, et al. Ciprofloxacin in primary prophylaxis of spontaneous bacterial peritonitis: a randomized, placebo-controlled study. J Hepatol 2008;48(5):774–779
Grangé JD, Roulot D, Pelletier G, Pariente EA, Denis J, Ink O, et al. Norfloxacin primary prophylaxis of bacterial infections in cirrhotic patients with ascites: a double-blind randomized trial. J Hepatol 1998;29:430–436
Singh N, Gayowski T, Victor LY, Wagener MM. Trimethoprim-sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: a randomized trial. Ann Intern Med 1995;122:595–598
Rolachon A, Cordier L, Bacq Y, Nousbaum JB, Franza A, Paris JC, et al. Ciprofloxacin and long-term prevention of spontaneous bacterial peritonitis: results of a prospective controlled trial. Hepatology 1995;22:1171–1174
Lontos S, Shelton E, Angus PW, Vaughan R, Roberts SK, Gordon A, et al. A randomized controlled study of trimethoprim-sulfamethoxazole versus norfloxacin for the prevention of infection in cirrhotic patients. J Dig Dis 2014;15:260–267
Moreau R, Elkrief L, Bureau C, Perarnau JM, Thévenot T, Saliba F, et al. Effects of long-term norfloxacin therapy in patients with advanced cirrhosis. Gastroenterology 2018;155:1816–1827
Bauer TM, Follo A, Navasa M, Vila J, Planas R, Clemente G, et al. Daily norfloxacin is more effective than weekly rufloxacin in prevention of spontaneous bacterial peritonitis recurrence. Dig Dis Sci 2002;47:1356–1361
Zayed EM, Zaghla HS, Rady MA, Badra GA, Mohamed MS, Waked IA. Evaluation of different regimens of oral antibiotics in secondary prevention of spontaneous bacterial peritonitis in cirrhotic patients. Egypt Liver J 2011;1:69–72
Alvarez RF, Mattos AA, Corrêa EB, Cotrim HP, Nascimento TV. Trimethoprim-sulfamethoxazole versus norfloxacin in the prophylaxis of spontaneous bacterial peritonitis in cirrhosis. Arq Gastroenterol 2005;42(4):256–262
Ginés P, Rimola A, Planas R, Vargas V, Marco F, Almela M, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology 1990;12(4):716–724
Elfert A, Abo Ali L, Soliman S, Ibrahim S, Abd-Elsalam S. Randomized-controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 2016;28(12):1450–1454
Mostafa T, Badra G, Abdallah M. The efficacy and the immunomodulatory effect of rifaximin in prophylaxis of spontaneous bacterial peritonitis in cirrhotic Egyptian patients. Turk J Gastroenterol 2015;26:163–169
Soriano G, Guarner C, Teixidó M, Such J, Barrios J, Enríquez J et al. Selective intestinal decontamination prevents spontaneous bacterial peritonitis. Gastroenterology 1991;100(2):477–481
Fernández J, Ruiz del Arbol L, Gómez C, Durandez R, Serradilla R, Guarner C et al. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology 2006;131(4):1049–1056
Sandhu BS, Gupta R, Sharma J, Singh J, Murthy NS, Sarin SK. Norfloxacin and cisapride combination decreases the incidence of spontaneous bacterial peritonitis in cirrhotic ascites. J Gastroenterol Hepatol 2005;20(4):599–605
Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–81.
Flamm SL, Mullen KD, Heimanson Z, Sanyal AJ. Rifaximin has the potential to prevent complications of cirrhosis. Therap Adv Gastroenterol 2018;11:1756284818800307. https://doi.org/10.1177/1756284818800307.
Minuk GY, Hawkins K, Kaita KD, Wong S, Renner E, Minuk L et al. Daily ciprofloxacin treatment for patients with advanced liver disease awaiting liver transplantation reduces hospitalizations. Dig Dis Sci 2011;56(4):1235–12341. https://doi.org/10.1007/s10620-010-1456-2.
Facciorusso A, Papagiouvanni I, Cela M, Buccino VR, Sacco R. Comparative efficacy of long-term antibiotic treatments in the primary prophylaxis of spontaneous bacterial peritonitis. Liver Int 2019;39(8):1448–1458
Wang W, Yang J, Liu C, Song P, Wang W, Xu H, et al. Norfloxacin, ciprofloxacin, trimethoprim-sulfamethoxazole, and rifaximin for the prevention of spontaneous bacterial peritonitis: a network meta-analysis. Eur J Gastroenterol Hepatol 2019;31:905–910
Fiore M, Di Franco S, Alfieri A, Passavanti MB, Pace MC, Kelly ME, et al. Spontaneous bacterial peritonitis caused by Gram-negative bacteria: an update of epidemiology and antimicrobial treatments. Expert Rev Gastroenterol Hepatol 2019;13(7):683–692
Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol 2010;26(1):17–25
European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69(2):406–460
Lombardi A, Zuccaro V, Fagiuoli S, Bruno R. Prophylaxis of spontaneous bacterial peritonitis: is there still room for quinolones? J Hepatol 2019;70(5):1027–1028
Acknowledgements
None.
Funding
No funding received
Author information
Authors and Affiliations
Contributions
HS and PKM contributed equally. HS: Database search, screening, data extraction, Risk of Bias, manuscript writing, manuscript revision and final approval to the manuscript. PKM: Data analysis, manuscript writing, manuscript revision and final approval to the manuscript. VS: Conception, screening, data extraction, Risk of Bias, manuscript writing, manuscript revision, important intellectual content and final approval to the manuscript. BLB: Database search, screening, data extraction, Risk of Bias and final approval to the manuscript. SM: Data extraction, manuscript writing and final approval to the manuscript. DM: Screening and final approval of the manuscript. HSM: Intellectual content, manuscript revision and final approval to the manuscript. BM: Intellectual content, manuscript revision and final approval to the manuscript. UD: Intellectual content, manuscript revision and final approval to the manuscript. VSi: Intellectual content, manuscript revision and final approval to the manuscript
Corresponding author
Ethics declarations
Conflict of interest
Hariom Soni, Praveen Kumar-M, Vishal Sharma, Balaji L. Bellam, Shubhra Mishra, Dhruv Mahendru, Harshal S. Mandavdhare, Bikash Medhi, Usha Dutta, Virendra Singh declare that they have no conflict of interest
Ethical approval
Not applicable as this article, a systematic review, does not contain any human participants or animal experimentation performed by any of the authors.
Informed consent
Not applicable, see above.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
PROSPERO Registration No: CRD42020140547.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soni, H., Kumar-M, P., Sharma, V. et al. Antibiotics for prophylaxis of spontaneous bacterial peritonitis: systematic review & Bayesian network meta-analysis. Hepatol Int 14, 399–413 (2020). https://doi.org/10.1007/s12072-020-10025-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10025-1