Abstract
Background
Regulatory T cell (Treg) plays an essential role in regulating anti-tumor immunity. The aim of this study was to investigate the effect of transarterial chemoembolization (TACE) on Treg in hepatocellular carcinoma (HCC) patients.
Method
The frequency of peripheral blood Tregs in 27 HCC patients who underwent TACE were measured at baseline and 1 month after TACE. The frequency of peripheral blood Tregs at baseline were compared with those in 23 healthy controls. Tregs were further classified into three subpopulations [Treg (I), Treg (II), Treg (III)] based on expression levels or markers and their function. The patients were divided into two groups according to tumor response after TACE; complete response group and incomplete response group. The correlations between the frequency of Treg and clinical factors were analyzed.
Results
The frequency of Treg in HCC patients (7.52%) was significantly higher than in healthy controls (4.99%) at baseline. Regarding Treg subpopulations, the frequency of Treg (II) was significantly higher in HCC patients (2.51%) than in healthy controls (0.60%). In comparison of Treg numbers at baseline and post-TACE by tumor response, the change of Treg (III) in complete response group from baseline to post-TACE was significantly decreased (63.8 → 53.2/mm3). Patients with a high post-TACE Treg (III) (3.8 months) exhibited a significantly shorter median time to progression than those with a low post-TACE Treg (III) (11.6 months). In multivariate analyses, hypoalbuminemia (hazard ratio 3.324; 95% CI 1.098–10.063, p = 0.034) and high post-TACE Treg (III) (hazard ratio 3.080; 95% CI 1.091–8.696, p = 0.034) were significant factors for associating with progression.
Conclusions
The frequency of Tregs in HCC patients was significantly higher than in healthy controls. In addition, patients with a high post-TACE Treg (III) exhibited a significantly lower progression-free survival rate than those with a low post-TACE Treg (III).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and is the third most common cause of cancer-related death. Although remarkable developments in HCC treatment and diagnosis have improved patient outcomes, treatment remains very challenging. Transarterial chemoembolization (TACE) is widely used and recommended treatment modality for patients with unresectable tumors, especially those with intermediate stage of HCC. However, it has resulted in only a 23% improvement in the 2-year survival compared with conservative care [1]. Therefore, identifying and establishing alternative approaches for the intermediate stage of HCC are of great interest.
The liver has been known as an organ with predominant innate immunity. Innate immune lymphocytes among liver cells are at least five times high to the percentages among spleen or peripheral blood. It is well known that viral hepatitis becomes chronic when immune cell function deteriorates [2]. During viral infection, T cells are important in terms of virus removal [2, 3]. Hepatitis infection becomes chronic when the virus fails to be eliminated by poorly effective T cells [2, 3]. Recent studies regarding tumor microenvironment have shown that several immune cells play an important role in cancer development and growth. Lack of anti-cancer immunity is associated with tumor growth, poor viral clearance, and other negative effects of cancer. Among them, regulatory T cell (Treg) is known to play essential roles in immune homeostasis and anti-tumor immunity. Treg is a subgroup of CD4+ T cells and suppress the activation, proliferation, differentiation as well as effector functions, of many types of immune cells, including T, B, NK, and dendritic cells [4,5,6]. Treg was first reported by Sakaguchi et al. in 1995 [7], and they further classified into three distinct subpopulations based on expression levels or markers and their functions [8]. They are: (1) CD45RA+Foxp3low Treg (Treg (I)), designated as naïve or resting Treg; (2) CD45RA−Foxp3high Treg (Treg (II)), designated as effector or activated Treg; (3) CD45RA−Foxp3low Treg (Treg (III)), which do not possess suppressive activity, but can secrete pro-inflammatory cytokines. Tregs have been observed in peripheral blood and tumor tissues and associated with negative outcomes in patients with several type of cancer, including acute lymphoblastic leukemia, breast cancer, pancreatic cancer, prostate cancer, head and neck cancer, and cervical cancer [9,10,11,12,13]. Although the roles of Tregs in various cancers have been studied, the activities of the various Treg subpopulations are unclear.
The studies on the roles of immune cells in HCC microenvironment have sought to expand understanding of HCC and to improve the existing HCC treatments [14,15,16,17]. HCC immunotherapy, which requires knowledge of the immunological background of HCC, has shown promising results in several recent trials [18, 19]. In addition, HCC immunotherapy in combination with the conventional locoregional treatments has been evaluated [19]. Because TACE is currently widely used for local treatment of HCC, its effects on the immune system should be determined. Several immunologic markers of the prognosis after TACE have been discovered [14, 20, 21], but a few studies have focused on Tregs. In addition, prior studies of the roles of Tregs in HCC have reported contradictory results [17]. Therefore, this study investigated the effects of TACE on Treg and its subpopulations in HCC patients.
Materials and methods
Patients and healthy controls
A total of 35 treatment-naïve patients with primary HCC treated with TACE and agreed to provide blood samples before and after TACE from December 2010 to December 2012 at Severance Hospital of Yonsei University College of Medicine, Seoul, Korea were enrolled in this study. The diagnosis of HCC for all patients was confirmed by clinical criteria based on the guidelines proposed by the Korea Liver Cancer Study Group [22]. Among them, eight patients whose cell viability in blood sample was low were excluded, and finally, 27 patients were used for the analysis of the study. The exclusion criteria were as follows: (1) inadequate target lesion (infiltrative pattern, non-arterial enhancement, or largest lesion < 1 cm); (2) presence of an additional primary malignancy in other organ; (3) presence of extrahepatic tumor lesions or tumor invasion to the main portal vein; (4) Child–Pugh class C; and (5) presence of uncontrolled functional or metabolic disease. Patients gave written informed consent before joining the study, and the study protocol, approved by the Institutional Review Board of Severance Hospital, conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The study also included 23 healthy controls.
Treatment method
Before the TACE procedure, angiography of the superior mesenteric and hepatic artery was performed to assess portal vein patency, vascular anatomy, and vascularity of the tumor. Conventional TACE was performed by selective infusion of a mixture of 5 ml iodized oil contrast medium (lipiodol; Guerbet; Bloomington, IN, USA) and either 50 mg Adriamycin or cisplatin at 2 mg/kg body weight in a subsegmental or segmental branch of the feeding arteries, followed by embolization using gelatin sponge particles (Cutanplast; Mascia Brunelli S.p.a, Milano, Italy). Embolization was performed until stasis was achieved in the second- or third-order branches of the right or left hepatic artery.
Follow-up assessment
A contrast-enhanced CT of the liver was performed 4–5 weeks after TACE to assess the effect of embolization on the tumor. Then, additional CT scans to monitor tumor recurrence were performed every 1 month for 6 months, every 3 months for 1 year, and every 6 months thereafter. The mRECIST criteria were applied to the imaging judgement of the tumor response. In addition, all imaging examinations were performed by two trained radiologists. When relapse of the treated lesions and/or new hepatic lesions were detected, patients were advised to receive repeated TACE if their liver functions were satisfactory and there were no contraindications. At each CT scan, tumor markers including AFP and PIVKA-II and other laboratory tests were performed. The patients were divided into two groups according to tumor response and repeated TACE. Patients who were completely treated without viable portion on the first CT after TACE and followed up for 6 months without recurrence or additional treatment were defined as the complete response group. Otherwise, patients were classified as the incomplete response group. All patients were followed from the date of TACE up to December 31, 2014, or up to the time of death.
Isolation of lymphocytes from peripheral blood and antibodies used in the study
Fresh heparinized peripheral blood samples from the 35 patients were obtained out the day before and 1 month after TACE. Blood was drawn into 4.5 ml EDTA tubes. Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood by Ficoll-Paque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation and cryopreserved until use.
The following fluorochrome-conjugated monoclonal antibodies were used for flow cytometry: anti-CD3-A700; anti-CD4-PerCP/Cy5.5; anti-CD45RA-APC/H7; anti-CD14-PE/TR; anti-CD19-PE/TR; anti-CD39-FITC; anti-TIGIT-PE/Cy7; anti-Foxp3-PE (eBioscience, San Diego, CA, USA), anti-CD8-V500; anti-CD25-BV650; anti-CD127-BV786; anti-CD152 (CTLA-4)-APC (BD Biosciences, San Jose, CA, USA), and anti-PD-1-V450 (Biolegend, San Diego, CA, USA).
Surface and intracellular staining and flow cytometric analysis of PBMCs
PBMCs were stained with fluorochrome-conjugated antibodies against surface markers for 30 min on ice and then washed. Dead cells were excluded by staining with either a Live/Dead fixable cell stain kit (Invitrogen, Carlsbad, CA, USA) or ethidium monoazide (Invitrogen). For intracellular staining, surface-stained cells were permeabilized using Foxp3 staining buffer kit (eBioscience) according to the manufacturer’s instructions, and were further stained for intracellular proteins of Foxp3 and CTLA-4. Flow cytometry was performed on an LSR II instrument using FACS Diva software (BD Biosciences), and the data were analyzed using FlowJo software (Treestar, San Carlos, CA, USA).
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Science (SPSS 22 for Windows; SPSS Inc., Chicago, IL, USA) or GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA, USA). Baseline clinical and tumor characteristics were presented as median (range) or n (%) as appropriate. All immunological cell fractions were expressed as median (range) or mean ± standard deviation (SD). Survival curves and survival rates were calculated using the Kaplan–Meier method and compared using Cox’s proportional hazard model. Survival was measured from the date of TACE to December 31, 2014, or up to the time of death. The adjusted hazard ratio (HR) and its 95% confidence interval (CI) for each of the selected risk factors were calculated. Age (> 60 years vs. ≤ 60 years), tumor size (> 5 cm vs. ≤ 5 cm), serum albumin (< 3.8 g/dl vs. ≥ 3.8 g/dl), and AFP (> 200 ng/ml vs. ≤ 200 ng/ml) were used as binary variables in the Cox models using the cut-offs. The cut-off value for the analysis for the clinical implications of Treg (low Treg vs. high Treg) was the mean of all patients’ data. Variables with p < 0.05 were defined as statistically significant.
Results
Baseline characteristics of patients
Patients’ clinical and tumor characteristics are presented in Table 1. The median age was 60.4 years and 21 (77.8%) patients were male. Most HCCs were related to hepatitis viral infection, HBV (n = 16, 59.3%), and HCV (n = 7, 25.9%). Most patients had preserved liver function with Child–Pugh class A (n = 23, 85.2%). The median size of the largest tumor was 3.07 cm, and 15 (55.6%) patients had multifocal lesions. TNM stage was divided as follows: I (n = 5, 18.5%), II (n = 13, 48.1%), III (n = 8, 29.6%), and IVa (n = 1, 3.7%). The median proportion of baseline CD4+ T cells among peripheral lymphocytes was 64.53% and baseline Treg frequency among peripheral CD4+T cells was 7.52%. Liver-related biochemical tests are also described in Table 1.
Comparison of Treg in HCC patients and healthy controls
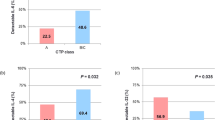
First, the frequency of Treg in the PBMC was measured by flow cytometry (Fig. 1a). As shown in Fig. 1b, the frequency of Treg among CD4+ T cells in HCC patients was significantly higher than in healthy controls (7.52 ± 2.62% in HCC vs. 4.99 ± 1.49% in healthy controls, p < 0.001). In particular, in a Treg subpopulation defined by CD45RA and FoxP3 levels (Fig. 1c), the frequency of Treg (I) and Treg (III) among CD4+ T cells did not differ significantly between the two groups, but the frequency of Treg (II) among CD4+ T cells was significantly higher in HCC patients than in healthy controls (2.51 ± 1.08% in HCC vs. 0.60 ± 0.28% in healthy controls, p < 0.001). (Fig. 1d). In addition, the frequency of CTLA-4 ( +) Treg (II) cells among CD4+ T cells differed significantly between patients and healthy controls (1.16 ± 0.63% in HCC vs. 0.37 ± 0.20% in healthy controls, p < 0.001) (Fig. 1e).
Comparison of Treg between HCC patients (n = 27) and healthy controls (n = 23). a Gating strategy for measuring the frequency of CD4+CD25+Foxp3+Tregs; b frequency of CD4+CD25+Foxp3+Tregs in peripheral blood of HCC patients and healthy controls; c classification of Treg subpopulation defined by CD45RA and FoxP3 expressions; d frequency of Treg subpopulations in peripheral blood in HCC patients and healthy controls; e frequency of CTLA-4 (+) Treg subpopulations in peripheral blood in HCC patients and healthy controls. ***p < 0.001 and **p < 0.05
Comparison of Treg and its subpopulations at baseline and post-TACE by tumor response
Table 2 shows how the numbers of Treg and those of Treg subpopulations from baseline to post-TACE changed. The number of cells was calculated by multiplying the frequency by the total lymphocyte count. In all patients (n = 27), the average Treg number at baseline was 134.9 ± 74.7/mm3 and that post-TACE was 135.2 ± 73.4/mm3, thus, not significantly different (p = 0.969). However, although not statistically significant, the Treg numbers post-TACE (compared to baseline) decreased in the complete response group (138.5 ± 78.1 → 125.0 ± 73.2/mm3, p = 0.086) and increased in incomplete response group (128.7 ± 72.1 → 152.4 ± 74.3/mm3, p = 0.099), consistent with the change of Treg (III) numbers baseline and post-TACE. In the complete response group, the change of Treg (III) from baseline to post-TACE was statistically significant (63.8 ± 42.2 → 53.2 ± 31.9/mm3, p = 0.050).
Clinical implications of Treg on survival and progression
Next, the clinical implications of Treg at baseline and post-TACE in terms of survival and progression were investigated. The cut-off values for the analysis for the clinical implications of Treg and Treg (III) were defined by the mean of all patients’ data. As shown in Fig. 2a, overall survival rate differed significantly between the complete and incomplete response groups (2-year survival rate; 82.4% in complete groups vs. 44.4% in incomplete group, p = 0.010). However, overall survival rate did not differ significantly between those with high and low levels of Treg both at baseline and post-TACE (Fig. 2b). There were also no differences in distinguishing the overall survival rate between high and low levels of Treg (III) at both baseline and post-TACE (Fig. 2c).
Cumulative overall survival rate stratified by response and Treg levels at baseline and post–TACE. a Comparison of overall survival rates regarding response; b comparison of overall survival rates regarding the frequency of Treg at baseline and post-TACE; c comparison of overall survival rates regarding the frequency of Treg (III) at baseline and post-TACE
In terms of progression-free survival rate, there were no differences between patients with high Treg and those with low Treg at both baseline and post-TACE (Fig. 3a). Although the progression-free survival rate also did not differ between patients with low baseline Treg (III) and those with high baseline Treg (III), patients with a high post-TACE Treg (III) exhibited a significantly shorter median time to progression than those with a low post-TACE Treg (III) (median time to progression; 3.8 vs. 11.6 months, p = 0.038) (Fig. 3b).
Cumulative progression-free survival rate stratified by response and Treg levels at baseline and post-TACE. a Comparison of progression-free survival rates regarding the frequency of Treg at baseline and post-TACE; b comparison of progression-free survival rates regarding the frequency of Treg (III) at baseline and post-TACE
Cox’s proportional analysis to explore the predictive utilities of multiple clinical factors in terms of both overall survival and progression rate after TACE was performed. On univariate analysis, hypoalbuminemia (< 3.8 g/dl) and high post-TACE Treg (III) were significantly associated with a high progression rate. On multivariate analysis, hypoalbuminemia (hazard ratio 3.324; 95% CI (1.098–10.063), p = 0.034) and high post-TACE Treg (III) (hazard ratio 3.080; 95% CI (1.091–8.696), p = 0.034) were significant factors for associating with progression (Table 3).
Discussion
This study explored the effects of TACE on Treg in peripheral blood in HCC patients and, significantly, investigated for the first time the role of Treg subpopulations.
Tregs are a subgroup of CD4+ T cells characterized by expression of CD25; “forkhead or winged helix family of transcription factor P3” (Foxp3) is critical in terms of Treg development and function [23]. CD4+CD25+Foxp3+ Tregs suppress the activation, proliferation, differentiation, as well as effector functions of many types of immune cells, including T, B, NK, and dendritic cells [4,5,6]. Thus, Tregs maintain self-tolerance and regulate the immune response in both the physiological and diseased state.
High Treg numbers are often associated with poor survival of those with breast and lung cancer, and melanomas [17, 24]. It has been hypothesized that Treg compromise anti-tumor effects, worsening outcomes. On the other hand, high Treg levels improve the prognosis of those with other cancers including colorectal and oropharyngeal cancer, and hematological malignancies [25]. These different results may reflect Treg heterogeneity; different Treg may influence tumors differently. Recently, it has been suggested that Treg plays dual roles in cancer development and progression [26]. In particular, cancers in which Treg appears to play positive roles for anti-cancer immunity are principally associated with chronic exposure to microorganisms and the microbial flora, which greatly influence cancer development and progression. Therefore, Treg may suppress tumor-promoting inflammatory reactions initiated by infectious agents [27].
In HCC patients, several studies have found that high Treg levels were associated with poor prognosis [28, 29]. However, the question of whether such levels are prognostic remains controversial. Previous studies on Treg of HCC patients included patients treated in various ways and explored Tregs of different sites, perhaps explaining the contradictory results. Previous study reported that higher intratumoral and peripheral blood Treg levels were associated with poorer prognosis, whereas a high peritumoral Treg level did not affect prognosis [17]. Many studies on Treg of HCC patients evaluated intratumoral Tregs only; a few studies have assessed Tregs of peripheral blood [17]. All studies on intratumoral Tregs used tumor tissue collected during surgeries such as resection. Surgical intervention is considered curative for HCC but only a few patients meet the surgical indications; most patients are diagnosed with advanced HCC. TACE is a nonsurgical intervention for patients with intermediate or even advanced HCC, and is the most commonly used treatment modality in clinical practice. It is difficult to obtain tumor tissue and to measure intratumoral Treg levels because patients scheduled for TACE do not need to be subjected to liver biopsy for diagnosis or treatment. A recent meta-analysis concluded that Tregs in peripheral blood are connected to intratumoral Treg in HCC [17]. The authors posited that because immune escape of tumors occurs in both local immunity and systemic immunity, Tregs in peripheral blood are crucial in the immunosuppression of the whole immune system. In addition, Tregs of peripheral blood are likely to be useful clinical markers; they can be detected noninvasively and monitored in real time. More studies on Treg in the peripheral blood of HCC patients are needed; the results of this study are meaningful.
In this study, patients with HCC had higher Treg frequency than healthy controls, consistent with the previous reports [15, 16, 30]. On the further analysis, by Treg subpopulations, the frequency of Treg (II) cells, which exert profoundly suppressive actions, was significantly higher and the frequency of Treg (II) with CTLA-4(+), an immunosuppressive marker, was also significantly higher in HCC patients compared to healthy controls. Previous studies have reported that tumor cells can evoke the proliferation of immunosuppressive cells including Tregs, which prevent the maturation of antigen presenting cells as well as presentation of tumor antigen [31, 32]. Therefore, the higher frequency of Tregs in HCC patients compared to healthy controls may be due to the improvement of Treg proliferation by tumor cells to avoid attack by the host immune system. In particular, it can be understood from this background that the frequency of Treg (II), which displayed the greatest suppressive activity among the Treg subpopulations, was higher in HCC patients than in healthy controls. Liao et al. compared Treg frequency before and 1 month after TACE and reported that Treg frequency decreased significantly after TACE [15]. Xiong et al. divided patients into two groups in terms of the response to TACE, and compared Treg frequency at baseline and 1 month after TACE in both groups [16]. In this study, although statistical significance was not attained, Treg number decreased in those who exhibited a complete response to TACE and increased in those who did not. In addition, Treg (III) number decreased significantly in the complete response group at post-TACE. Such clinical significance was also apparent on survival analysis; the progression-free survival rate was significantly lower in patients with high post-TACE Treg (III) levels than others. Also, the high post-TACE Treg (III) was one of significant factors associating with progression in multivariate analysis.
It is known that Treg (III) cells do not exhibit a suppressive activity, but do secrete pro-inflammatory cytokines. Thus, the function of Treg (III) cells appears to be unlike that of conventional Treg, which primarily exerts profound suppression. Possible explanation as to why Treg (III) frequencies were high in bad responders was advanced as follows. The increase in Treg (III) levels may reflect repair of tissue damage after TACE (which is known to injure liver tissue in an ischemic or toxic manner), and that the extent of repair represented by deterioration of liver function is prognostic [33]. This hypothesis is supported by the increased numbers of Treg (III) cells in patients with acute-to-chronic liver failure correlating with severe liver injury [34]. A recent study by Choi et al. reported that TNF-producing Tregs from patients with acute hepatitis A were associated with severe liver injury [35]. They founded these Tregs showed altered functions with low Foxp3 expression and reduced suppressive activity. These features of TNF-producing Tregs seemed to be similar with those of Treg (III), suggesting that TNF-producing Tregs and Treg (III) may be the same subpopulation. The existence of TNF-producing Tregs is also supported by several previous reports regarding increased levels of various cytokine including TNF-alpha after TACE, suggesting a mechanism by which hepatic injury enhances the proliferation of liver macrophage to repair [36, 37].
Treg subpopulations have not been well studied and their roles are poorly understood. To the best of our knowledge, this is the first study to analyze Treg subpopulations in HCC patients. These findings differ from the previous reports on HCC patients treated via TACE in that it was evaluated Foxp3 status; Foxp3 is an important transcription factor used to define a Treg subpopulation. Nevertheless, further studies on the molecular biological mechanisms in play are needed, and prospective studies with larger number of patients and more homogenous groups in terms of underlying disease and cancer stage should be conducted to evaluate the clinical usefulness of Treg and its subpopulations in HCC.
In conclusion, the frequency of Tregs in HCC patients was significantly higher than in healthy controls. In addition, patients with a high post-TACE Treg (III) exhibited a significantly lower progression-free survival rate than those with a low post-TACE Treg (III).
Abbreviations
- Treg:
-
Regulatory T cell
- TACE:
-
Transarterial chemoembolization
- HCC:
-
Hepatocellular carcinoma
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–188.
Jung MK, Shin EC. Regulatory T cells in hepatitis B and C virus infections. Immune Netw. 2016;16:330–6.
Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509–23.
Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–32.
Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–38.
Mahnke K, Enk AH. Dendritic cells: key cells for the induction of regulatory T cells? Curr Top Microbiol Immunol. 2005;293:133–50.
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64.
Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911.
Wu CP, Qing X, Wu CY, Zhu H, Zhou HY. Immunophenotype and increased presence of CD4(+)CD25(+) regulatory T cells in patients with acute lymphoblastic leukemia. Oncol Lett. 2012;3:421–4.
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61.
Zhao E, Wang L, Dai J, Kryczek I, Wei S, Vatan L, et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. 2012;1:152–61.
Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–72.
Adurthi S, Mukherjee G, Krishnamurthy H, Sudhir K, Bafna UD, Umadevi K, et al. Functional tumor infiltrating TH1 and TH2 effectors in large early-stage cervical cancer are suppressed by regulatory T cells. Int J Gynecol Cancer. 2012;22:1130–7.
Liao Y, Wang B, Huang ZL, Shi M, Yu XJ, Zheng L, et al. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma: a prospective study. PLoS ONE. 2013;8:e60444.
Liao J, Xiao J, Zhou Y, Liu Z, Wang C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol Med Rep. 2015;12:6065–71.
Xiong B, Feng G, Luo S, Liang H, Qiu L, Zheng C, et al. Changes of CD4(+) CD25 (+) regulatory T cells in peripheral blood in patients with hepatocellular carcinoma before and after TACE. J Huazhong Univ Sci Technol Med Sci. 2008;28:645–8.
Sun L, Xu G, Liao W, Yang H, Xu H, Du S, et al. Clinicopathologic and prognostic significance of regulatory T cells in patients with hepatocellular carcinoma: a meta-analysis. Oncotarget. 2017;8:39658–72.
Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(1383–1391):e1386.
Kudo M. Immuno-oncology in hepatocellular carcinoma: 2017 update. Oncology. 2017;93(Suppl 1):147–59.
Xue TC, Jia QA, Ge NL, Chen Y, Zhang BH, Ye SL. Imbalance in systemic inflammation and immune response following transarterial chemoembolization potentially increases metastatic risk in huge hepatocellular carcinoma. Tumour Biol. 2015;36:8797–803.
Tampaki M, Doumba PP, Deutsch M, Koskinas J. Circulating biomarkers of hepatocellular carcinoma response after locoregional treatments: new insights. World J Hepatol. 2015;7:1834–42.
Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. https://koreamed.org/article/1005KJHEP/2009.15.3.391
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61.
Jiang D, Gao Z, Cai Z, Wang M, He J. Clinicopathological and prognostic significance of FOXP3+ tumor infiltrating lymphocytes in patients with breast cancer: a meta-analysis. BMC Cancer. 2015;15:727.
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92.
Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–6.
Frydrychowicz M, Boruczkowski M, Kolecka-Bednarczyk A, Dworacki G. The dual role of Treg in cancer. Scand J Immunol. 2017;86:436–43.
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39.
Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–11.
Li F, Guo Z, Lizee G, Yu H, Wang H, Si T. Clinical prognostic value of CD4+CD25+FOXP3+regulatory T cells in peripheral blood of Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma patients. Clin Chem Lab Med. 2014;52:1357–65.
Cao M, Cabrera R, Xu Y, Firpi R, Zhu H, Liu C, et al. Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+)CD25(+) regulatory T cells. Lab Investig. 2007;87:582–90.
Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F. Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity. Cancer Immunol Immunother. 2008;57:1579–87.
Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Muller C, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261–73.
Zhang M, Zhou J, Zhao T, Huang G, Tan Y, Tan S, et al. Dissection of a circulating and intrahepatic CD4(+)Foxp3(+) T-cell subpopulation in chronic hepatitis B virus (HBV) infection: a highly informative strategy for distinguishing chronic HBV infection states. J Infect Dis. 2012;205:1111–20.
Choi YS, Jung MK, Lee J, Choi SJ, Choi SH, Lee HW, et al. Tumor necrosis factor-producing T-regulatory cells are associated with severe liver injury in patients with acute hepatitis A. Gastroenterology. 2018;154:1047–60.
Itoh Y, Okanoue T, Ohnishi N, Nishioji K, Sakamoto S, Nagao Y, et al. Hepatic damage induced by transcatheter arterial chemoembolization elevates serum concentrations of macrophage-colony stimulating factor. Liver. 1999;19:97–103.
Wang Y, Zheng C, Liang B, Zhao H, Qian J, Liang H, et al. Hepatocellular necrosis, apoptosis, and proliferation after transcatheter arterial embolization or chemoembolization in a standardized rabbit model. J Vasc Interv Radiol. 2011;22:1606–12.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Hana Park, Jae Hyung Jung, Min Kyung Jung, Eui-Cheol Shin, Simon Weonsang Ro, Jeon Han Park, Do Young Kim, Jun Yong Park, Kwang-Hyub Han declare that they have no conflict of interest.
Informed consent
Patients gave written informed consent before joining the study, and the study protocol, approved by the Institutional Review Board of Severance Hospital, conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, H., Jung, J.H., Jung, M.K. et al. Effects of transarterial chemoembolization on regulatory T cell and its subpopulations in patients with hepatocellular carcinoma. Hepatol Int 14, 249–258 (2020). https://doi.org/10.1007/s12072-020-10014-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10014-4