Abstract
At the time of hepatocellular carcinoma (HCC) diagnosis, patients are most often at an advanced stage; however, the current treatment regimens remain unsatisfactory. Thus, novel and more powerful therapeutic approaches for advanced HCC are urgently required. Exacerbation of immunotolerant signals and/or escaping immunosurveillance leads to the development of HCC, which appears to be a rational reason to use immunotherapy to restore anticancer immunity. Several novel immunotherapeutic methods, including the use of immune checkpoint inhibitors, new types of immune cell adoption [e.g., chimeric antigen receptor T cell (CAR-T), TCR gene-modified T cells and stem cells], and microRNAs have been used in clinical trials for the treatment of HCC. However, some crucial issues remain to be addressed for such novel immunotherapy techniques. Finally, immunotherapy is now standing on the threshold of great advances in the fight against HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary liver cancer is the second leading cause of cancer mortality and the fifth most common cancer worldwide. HCC is the most well-studied subtype and accounts for 85–90% of all primary liver cancers. Most HCCs are associated with a cirrhotic liver, with the primary cause being a chronic hepatitis B (HBV) or C (HCV) virus infection, followed by other etiologies, including alcohol consumption and fatty liver disease associated with metabolic syndrome [1]. In particular, alcoholic cirrhosis may become a leading cause of HCC in the future since it occupies approximately 47% of liver cirrhosis worldwide. Approximately 383,000 individuals die from liver cancer each year in China, which accounts for 51% of the deaths caused by liver cancer worldwide. In addition, as many as 80% of HCC cases in China are attributed to chronic HBV infection [2]. While patients in the early stages of the disease have a relatively good prognosis with a 5-year survival greater than 70%, the majority of HCC patients are diagnosed with late stage disease resulting in an overall 5-year survival rate less than 16% [3]. Immunological pathogenesis has raised additional concerns in HCC and immune therapy has gradually become a potentially powerful treatment for such advanced HCC patients.
Traditional and other therapeutic methods (Fig. 1)
According to the treatment guidelines, surgical resection, orthotopic liver transplantation, and percutaneous ablation can only be applied to less than 30% of early stage HCC patients [4]; however, most HCC patients are usually too late to see doctors when they are at an advanced status due to difficulties in making an early diagnosis. The molecularly targeting drug, sorafenib, a multiple tyrosine kinase inhibitor, was the first systemic agent approved by the FDA associated with the first-line treatment of patients with unresectable HCC [5]. Lenvatinib, an oral tyrosine kinase inhibitor, subsequently received FDA approval for the treatment of chemotherapy-naive HCC patients in July 2018 as another first-line treatment strategy [6]. In 2017, regorafenib, an oral kinase inhibitor that targets multiple protein kinases was approved by the FDA as a second-line treatment for advanced HCC after failing to respond to or tolerate sorafenib [7]. Other treatment regimens, including gene therapy, cytotoxic chemotherapy, radiotherapy, hormonal therapy, and Chinese herbal therapy [8] remain unsatisfactory for the treatment of HCC. Recently, immune therapy has been considered a potentially powerful treatment for such advanced HCC patients.
From the recommended guidelines to ongoing novel immunotherapy methods for advanced HCC patients. By following the recommended guidelines, less than 30% of HCC patients benefit from surgical resection, orthotopic liver transplantation, or percutaneous ablation. Other treatment regimens, including cytotoxic chemotherapy, radiotherapy, hormonal therapy, and herbal therapy remain unsatisfactory treatments for the disease. Recently, immune therapy has been considered to be a potentially powerful treatment for advanced HCC patients
Immune disorder in HCC: rationale for immunotherapy (Fig. 2)
The liver is the largest immune organ in human body; under physiologic conditions, it plays a protective role by promoting immunotolerance [9]. However, the exacerbation of immunotolerant signals or escaping from immunosurveillance, inevitably leads to the development of HCC [10]. Therefore, immunotherapies appear to be an appropriate method of activating latent anticancer immunity.
Innate and adaptive immune cells
The immune system can be divided into innate and adaptive immune responses, which can identify and destroy nascent tumor cells in a process termed cancer immune surveillance, which functions as an important defense against cancer; however, the immunosuppressive cancer environment (including HCC) substantially impacts the frequency and function of innate and adaptive immune cells, which finally lead to progression and metastasis in cancer patients. Since some reviews [11, 12] have already summarized the relationship between innate and adaptive immune cells and HCC, this review does not repeat such discussion here.
Cytokines and chemokines
Cytokines comprise an extremely important part of the immune system as a response to foreign pathogens, which can regulate the growth, differentiation, and activation of immune cells. Since previous reviews provide a comprehensive description regarding the relationship between cytokines and hepatocarcinogenesis [13], we only mention cytokines that have been in the spotlight within the past 2 years. Transforming growth factor beta (TGF-β) can cause a migratory stemness phenotype, and a higher expression of TGF-β is predictive of poor disease prognosis in HCC patients [14]. Interleukin (IL)-6 could also enhance cancer stemness and promote HCC metastasis [15]. IL-22 plays a dual role shifting from hepatoprotective to carcinogenic functionality [16]. Other cytokines, such as IL-15, IL-35, and IL-18 have also been reportedly connected with the outcome of HCC patients [17].
Chemokines and their receptors have also received increased attention. Previous studies have reported higher levels of expression and a significant correlation between CXCL12–CXCR4 expression and tumor progression, metastasis, and a decreased survival rate in HCC patients [18]. A recent study reported that both CXCL12 and CXCR4 polymorphisms are associated with increased susceptibility to HCC development [19]. Other chemokine axes, including CXCL8 [interleukin 8 or chemokine (C-X-C motif) ligand 9], XCR1, CCR1, CXCL5, CXCR3, CCL3, CX3CL1 and CCL20 have also been reportedly involved in the development of HCC [20].
Immune checkpoint molecules
Programmed cell death protein-1(PD-1)/PD-1 ligand (PD-L1) The first protein identified as an immune checkpoint molecule was PD-1, which was discovered in 1992 by Tasuku Honjo [21]. In 2009, Gao et al. [22] first found that PD-L1 overexpression was significantly associated with tumor aggressiveness and postoperative recurrence in HCC patients. In 2011, Shi [23] found that the upregulation of PD-1 and PD-L1 promoted CD8(+) T-cell apoptosis and postoperative recurrence in HCC patients. Thereafter, the impaired function of CD8+ T cells, CD4+ T cells, and myeloid-derived suppressor cells (MDSCs)/macrophages in HCC patients were also reported to correlate with PD-1/PD-L1 expression [24]. In addition, several clinical studies have found that the over-expression of PD-1/PD-L, both in the circulation [25] and the liver tissue [26], are associated with a poor prognosis in HCC patients.
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) The relationship between CTLA-4 and HCC was first noted by its genetic susceptibility [27]. Further studies subsequently showed that tumor-derived Treg cells could inhibit DC function by CTLA-4 in HCC patients [28]. More recent studies have found that effector Tregs and CD8+ T cells strongly express CTLA-4 in NASH-related HCC, whereas PD-1 was only highly expressed in CD8+ T cells in HBV-related HCC patients [29].
T-cell immunoglobulin and mucin domain-3 (TIM-3) In 2002, TIM-3 was first identified as a molecule selectively expressed on IFN-γ-producing CD4+ T helper 1 (Th1) and CD8+ T cytotoxic 1 (Tc1) cells [30]. In 2012, Li et al. [31] were the first to report that the TIM-3/galectin-9 signaling pathway mediates T-cell dysfunction and could predict a poor prognosis in patients with hepatitis B virus-associated HCC (HBV-HCC) patients. In 2015, Yan et al. [32] found that TIM-3 expression was significantly increased in both peripheral blood monocytes and tumor-associated macrophages (TAMs) in patients with HCC, which was strongly correlated with higher tumor grades and the poor survival of patients with HCC. In 2018, Li et al. [33] found that highly elevated levels of soluble TIM-3 correlated with an increased HCC risk and poor survival of HBV-HCC patients.
MicroRNAs (miRNAs)
With the exception of functioning as oncogenes or tumor suppressors genes, miRNAs also play an immune modulatory role during HCC progression [34]. MiR-615-5p, miR-889 and miR-146a have been found to negatively regulate NK cell functions [35, 36], whereas miR-152 and miR-182 play the opposite roles for NK cells in HCC [37, 38]. MiR-214, miR-28-5p, and miR-98 regulate macrophage polarization, which was correlated with tumor metastasis, recurrence, and poor survival in HCC patients [39, 40]. In addition, miR-34a, hsa-miR-182-5p, hsa-miR-214-3p, and miR-125b have all been reported to regulate Treg cell function in HCC patients [41]. Moreover, miR-197 and miR-451 were reported to target the IL-6/STAT3 inflammatory signaling pathway [42, 43], while miR-449 and miR-542-3p targeted the TGF-β/Smad signaling pathway [44, 45], all of which were involved in the development of HCC.
Cancer immune subsets
The systematic interrogation of tumor-infiltrating lymphocytes is key to the development of immunotherapies and the prediction of their clinical responses in cancer. Recently, Thorsson and his colleagues performed an extensive analysis of 10,000 tumors comprising 33 diverse cancer types, and revealed six immune subsets associated with various cancers: wound healing; IFN-γ-dominant; inflammatory; lymphocyte-depleted; immunologically quiet and TGF-β-dominant [46], while the majority of HCCs are characterized as a lymphocyte-depleted immune subset. Together, these data provide a resource for understanding immune–tumor interactions, with implications for identifying methods of advancing research on immunotherapy.
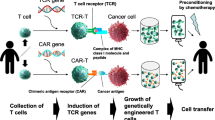
Current immunotherapeutic methods for HCC patients under clinical trial (Fig. 3)
Immune-based approaches focused on tumor vaccination, cytokines, non-specific T-cell activation, or adoptive cell transfer (e.g., DC and NKT) have been tested in HCC patients, however, the results were largely disappointing [47]. During the past few years, several novel immunotherapeutic methods, including immune checkpoint inhibitors used as monotherapy or combination therapy, new types of immune cell adoption, such as CAR-T, TCR gene-modified T cells and stem cells have been used in clinical trials for the treatment of HCC.
Clinical trials for immune therapy in HCC patients. Several novel immunotherapy methods, including immune checkpoint inhibitors that can be used as monotherapy or combination therapy, new types of immune cell adoption, such as chimeric antigen receptor T cells (CAR-T), TCR gene-modified T cells and stem cells, and microRNAs have been used in clinical trials for the treatment of HCC, some of them are even got permitted from FDA
Immune checkpoint inhibitor (Table 1)
Anti-CTLA-4 antibodies
In 2013, tremelimumab [48] was first used to treat HCC patients and its safety profile and anti-tumor activity supported further investigation. In 2017, Duffy et al. [49] found that tremelimumab used in combination with tumor ablation is a potential new treatment for patients with advanced HCC; however, only a small subset of patients responded to treatment. Tremelimumab plus durvalumab combination therapy in a phase III trial (NCT03298451) is currently underway to evaluate treatment efficacy. Ipilimumab, another anti-CTLA-4 antibody, is also being assessed in clinical trials for HCC patients (Table 1).
Anti-PD-1 antibodies
The positive results of CheckMate 040 (nivolumab) in HCC patients were published in The Lancet [50]. Primarily based on the data from this paper, the United States Food and Drug Administration approved nivolumab as second-line treatment agent for HCC on September 22, 2017. One case report suggested that metastatic HCC was responsive to pembrolizumab (a PD-1 inhibitor) following the failure of sorafenib [51]. Recently, combination therapies (e.g., nivolumab combined with Y-90 radioembolization [52]) may further enhance the anti-tumoral effects. A phase III Keynote-240 trial (NCT02702401) has recently found that the therapy did not meet its co-primary endpoints compared with the placebo. Other approaches involving combination therapy with a PD-1 blockade are currently ongoing (Table 1).
Anti-PD-L1 antibodies
The safety and activity of anti-PD-L1 antibody treatment has been proven in patients with advanced cancer [53]. In 2018, Liu et al. [54] provided a novel methodology to evaluate PD-L1 expression in the tumor microenvironment, which may help to select patients who would benefit from anti-PD-1/PD-L1 immunotherapies. All types of anti-PD-L1 antibodies (durvalumab, atezolizumab, and avelumab) are under investigation for their application in HCC patients (Table 1).
Other potential antibodies
Recently, a phase II clinical trial assessing the use of TSR-022 (anti-TIM3 antibodies) plus anti-PD-1 antibodies to treat advanced HCC has been registered (NCT03680508). Other inhibitory checkpoint molecules [e.g., lymphocyte-activation gene 3 (LAG-3), B- and T-lymphocyte attenuator (BTLA), and glucocorticoid-induced tumor necrosis factor receptor (GITR)] have also been found to be closely correlated with HCC progression, which may provide novel targets for the treatment of liver cancer [55].
Adoptive cellular transfusion
Cytokine-induced killer cells (CIKs)
In 2000, autologous lymphocytes activated in vitro were capable of lowering the frequency of recurrence following surgery for HCC [56]. From 2002, Prof. Wang focused on the anti-tumor activity of CIKs, and was the first to initiate CIK therapy for primary HCC patients in a phase I clinical trial [57], and found that the symptoms and characteristics of HCC patients were relieved without any major side effects. Thereafter, a series of classic randomized controlled trials for CIK were initiated, the results of which further confirmed the high treatment efficacy of CIK for HCC patients [58].
Antigen-specificity of HCC immunity
Several tumor-associated antigen (TAA)-specific T cells that targeted alpha-fetoprotein, glypican-3 (GPC3), melanoma-associated gene-A1, and New York-esophageal squamous cell carcinoma-1, respectively, have been regarded as potential therapeutic methods for HCC [59]. However, immunosuppressive mechanisms (e.g., immune checkpoint inhibitory molecules) lead to the above-mentioned specific T-cell exhaustion. Thus, blocking PD-L1, TIM3, and LAG3 might boost TAA-specific T-cell responses for the treatment of HCC [60]. Furthermore, some immunotherapies used to treat HCC patients have been found to favor the development of neoantigen-specific T cells that further enhance the anti-tumor response, leading to tumor shrinkage [61].
CAR-T cells
CAR-T cells are genetically engineered T cells achieved through the introduction of a chimeric antigen receptor. In 2014, GPC3-targeted CAR-T cells were first reported to specifically kill GPC3-positive HCC cells in vitro, and could significantly prolong the survival of HCC xenograft model [62]. In 2017, dual-targeted CAR-T cells co-expressing GPC3 and asialoglycoprotein receptor 1 (ASGR1), could exert superior anticancer activity [63]. Recently, CAR-T-directed CD133 (CART-133) was first tested in a phase I clinical study. The results showed the feasibility, controllable toxicity, and effective activity for its use in treating CD133-postive and late-stage metastasis of HCC patients [64]. Other targeted CAR-T therapies against liver cancer are currently under investigation [65].
TCR gene-modified T cells
In 2015, HBV-specific T-cell receptor (TCR)-modified T cells were constructed from Prof. Antonio’s lab, and a case report confirmed their feasibility and anti-tumor efficacy against HCC [66]. Thereafter, two clinical trials in China were initiated that involved the transfusion of escalating doses of HBV/TCR-T to treat (NCT02719782) and prevent (NCT02686372) the recurrence of HCC. In 2016, Spear et al. [67] found that hepatitis C-associated HCC could be efficiently treated by TCR gene-modified T cells. Additionally, in a recent study, Zhu et al. [68] identified AFP158-specific TCRs to have a substantial potential to treat HCC tumors. A Phase I open label clinical trial (NCT03132792) evaluating the safety and anti-tumor activity of AFPc332-specific TCRs in HLA-A2-positive subjects with advanced HCC patients is currently recruiting subjects.
Stem cells
In 2012, Ning et al. [69] reported that cancer stem cell (CSC)-vaccinated hosts were capable of killing CSCs in vitro. Based on these findings, a clinical trial (NCT02089919), termed the CSC Vaccine Therapy for treating HCC patients was performed between February 2014 and February 2015 without publishing the results. In 2015, Wang et al. [70] conducted a phase I trial of autologous DCs pulsed with autologous irradiated tumor stem cells (TSC) to treat HBV-HCC patients; autologous DC-TSC was found to be safe and did not exacerbate HBV in these HCC patients. In addition, genetically engineered mesenchymal stem cells co-expressing IFN-γ and IL-10 [71] or GPC3/CD3 bispecific T-cell engager (GPC3-ENG) [72] could inhibit HCC both in vitro and in vivo.
Other potential immunotherapy targets
Increased regulatory T cells (Tregs) represented both a potential prognostic marker and a therapeutic target for HCC [73]. Thereafter, decreasing the frequency and suppressor function of circulating Tregs with cyclophosphamide can increase tumor-specific immune responses in patients with advanced HCC (NCT00396682) [74]. Other targets, including microRNAs (e.g., MRX34), natural killer cells, cell cycle inhibitors, and exosomes are currently undergoing investigation as novel immunotherapeutic targets for HCC treatment [75,76,77].
Perspective
Although most of novel immune therapeutic methods are currently in clinical trials, some exhibit substantial potential anti-tumor efficacy. The following crucial issues must be considered: (1) standardization in the preparation of immune products. There are several different cell-based immunotherapies, and the standard operating procedure (e.g., source of the cells, culture methods, administration route, dosage, treatment cycle, stability indicating assay, and storage conditions) must be considered for each. In addition, the method of achieving individualized treatment should also be established based on each specific product; (2) a standardized clinical immunotherapy protocol. The timing of immunotherapy initiation and optimal patients who might benefit from such novel immunotherapeutic methods are current issues. Moreover, which target or combination of targets is the best strategy remains a concern for physicians; and (3) further study is necessary to elucidate the related mechanisms that are involved in the tumor microenvironment, gut microbiome, and HCC genomic features, which may influence the failure of immunotherapy. Moreover, safety issues should also be closely monitored. Finally, armed with a new understanding and unprecedented opportunities, the field of immunotherapy is now standing on the threshold of great advances in the fight against HCC.
References
Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol 2015;9(6):765–779
Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology 2014;60(6):2099–2108
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63(1):11–30
Song P, Cai Y, Tang H, Li C, Huang J. The clinical management of hepatocellular carcinoma worldwide: a concise review and comparison of current guidelines from 2001 to 2017. Biosci Trends 2017;11(4):389–398
Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359(4):378–390
Kudo M, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391(10126):1163–1173
Bruix J, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389(10064):56–66
Allaire M, Nault JC. Advances in management of hepatocellular carcinoma. Curr Opin Oncol 2017;29(4):288–295
Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol 2018;26(36):247–277
Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol 2018;19(3):222–232
Nishida N, Kudo M. Immunological microenvironment of hepatocellular carcinoma and its clinical implication. Oncology 2017;92(Suppl 1):40–49
Ilan Y. Immune therapy for hepatocellular carcinoma. Hepatol Int 2014;8(Suppl 2):499–504
Gelu-Simeon M, Samuel D. Role of cytokine levels in assessment of prognosis and post-treatment outcome in hepatocellular carcinoma. Hepatol Int 2013;7(3):788–791
Malfettone A, et al. Transforming growth factor-beta-induced plasticity causes a migratory stemness phenotype in hepatocellular carcinoma. Cancer Lett 2017;392:39–50
Mi F, Gong L. Secretion of interleukin-6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma. Biosci Rep 2017. https://doi.org/10.1042/bsr20170181
Saalim M, et al. IL-22: a promising candidate to inhibit viral-induced liver disease progression and hepatocellular carcinoma. Tumour Biol 2016;37(1):105–114
Easom NJW, et al. IL-15 overcomes hepatocellular carcinoma-induced NK cell dysfunction. Front Immunol 2018;9:1009
Liu H, et al. Roles of chemokine receptor 4 (CXCR18) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol 2008;5(5):373–378
Qin LF, et al. CXCL12 and CXCR19 polymorphisms and expressions in peripheral blood from patients of hepatocellular carcinoma. Future Oncol 2018;14(13):1261–1271
Liang CM, et al. Chemokines and their receptors play important roles in the development of hepatocellular carcinoma. World J Hepatol 2015;7(10):1390–1402
Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11(11):3887–3895
Gao Q, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15(3):971–979
Shi F, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011;128(4):887–896
Long J, et al. Expression of programmed death ligand-1 and programmed death 1 in hepatocellular carcinoma and its clinical significance. J Cancer Res Ther 2018;14(Supplement):S1188–S1192
Zeng Z, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One 2011;6(9):e23621
Jung HI, et al. Overexpression of PD-L1 and PD-L2 is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res Treat 2017;49(1):246–254
Gu X, et al. +49G > A polymorphism in the cytotoxic T-lymphocyte antigen-4 gene increases susceptibility to hepatitis B-related hepatocellular carcinoma in a male Chinese population. Hum Immunol 2010;71(1):83–87
Chen X, Du Y, Hu Q, Huang Z. Tumor-derived CD4+ CD25+ regulatory T cells inhibit dendritic cells function by CTLA-4. Pathol Res Pract 2017;213(3):245–249
Inada Y, et al. Characteristics of immune response to tumor-associated antigens and immune cell profile in hepatocellular carcinoma patients. Hepatology 2019;69(2):653–665
Monney L, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002;415(6871):536–541
Li H, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012;56(4):1342–1351
Yan W, et al. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut 2015;64(10):1593–1604
Li F, et al. Highly elevated soluble Tim-3 levels correlate with increased hepatocellular carcinoma risk and poor survival of hepatocellular carcinoma patients in chronic hepatitis B virus infection. Cancer Manag Res 2018;10:941–951
Khan FS, Ali I, Afridi UK, Ishtiaq M, Mehmood R. Epigenetic mechanisms regulating the development of hepatocellular carcinoma and their promise for therapeutics. Hepatol Int 2017;11(1):45–53
Xie H, et al. microRNA-889 is downregulated by histone deacetylase inhibitors and confers resistance to natural killer cytotoxicity in hepatocellular carcinoma cells. Cytotechnology 2018;70(2):513–521
Xu D, Han Q, Hou Z, Zhang C, Zhang J. miR-146a negatively regulates NK cell functions via STAT1 signaling. Cell Mol Immunol 2017;14(8):712–720
Bian X, et al. Down-expression of miR-152 lead to impaired anti-tumor effect of NK via upregulation of HLA-G. Tumour Biol 2016;37(3):3749–3756
Abdelrahman MM, et al. Enhancing NK cell cytotoxicity by miR-182 in hepatocellular carcinoma. Hum Immunol 2016;77(8):667–673
Zhou SL, et al. miR-28-5p-IL-34-macrophage feedback loop modulates hepatocellular carcinoma metastasis. Hepatology 2016;63(5):1560–1575.
Li L, et al. MiR-98 modulates macrophage polarization and suppresses the effects of tumor-associated macrophages on promoting invasion and epithelial–mesenchymal transition of hepatocellular carcinoma. Cancer Cell Int 2018;18:95. https://doi.org/10.1186/s12935-018-0590-3
Chen L, et al. Special role of Foxp3 for the specifically altered microRNAs in regulatory T cells of HCC patients. BMC Cancer 2014;14:489. https://doi.org/10.1186/1471-2407-14-489
Wang H, et al. Reciprocal control of miR-197 and IL-6/STAT3 pathway reveals miR-197 as potential therapeutic target for hepatocellular carcinoma. Oncoimmunology 2015;4(10):e1031440
Liu X, Zhang A, Xiang J, Lv Y, Zhang X. miR-451 acts as a suppressor of angiogenesis in hepatocellular carcinoma by targeting the IL-6R-STAT3 pathway. Oncol Rep 2016;36(3):1385–1392
Sandbothe M, et al. The microRNA-449 family inhibits TGF-beta-mediated liver cancer cell migration by targeting SOX4. J Hepatol 2017;66(5):1012–1021
Zhang T, et al. Downregulation of miR-542-3p promotes cancer metastasis through activating TGF-beta/Smad signaling in hepatocellular carcinoma. Onco Targets Ther 2018;11:1929–1939
Thorsson V, et al. The immune landscape of cancer. Immunity 2018;48(4):812–830
Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2015;12(12):681–700
Sangro B, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59(1):81–88
Duffy AG, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66(3):545–551
El-Khoueiry AB, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389(10088):2492–2502
Truong P, Rahal A, Kallail KJ. Metastatic hepatocellular carcinoma responsive to pembrolizumab. Cureus 2016;8(6):e631
Wehrenberg-Klee E, Goyal L, Dugan M, Zhu AX, Ganguli S. Y-90 Radioembolization combined with a PD-1 inhibitor for advanced hepatocellular carcinoma. Cardiovasc Interv Radiol 2018;41(11):1799–1802
Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366(26):2455–2465
Liu CQ, et al. Expression patterns of programmed death ligand 1 correlate with different microenvironments and patient prognosis in hepatocellular carcinoma. Br J Cancer 2018;119(1):80–88
Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res 2018;37(1):110
Takayama T, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000;356(9232):802–807
Shi M, et al. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol 2004;10(8):1146–1151
European Association for the Study of the Liver. Electronic address IEEE, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69(1):182–236
Flecken T, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8 + T-cell responses in hepatocellular carcinoma. Hepatology 2014;59(4):1415–1126
Zhou G, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology 2017;153(4):1107–1119
Desrichard A, Snyder A, Chan TA. Cancer neoantigens and applications for immunotherapy. Clin Cancer Res 2016;22(4):807–812
Gao H, et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 2014;20(24):6418–6428
Chen C, et al. Development of T cells carrying two complementary chimeric antigen receptors against glypican-3 and asialoglycoprotein receptor 1 for the treatment of hepatocellular carcinoma. Cancer Immunol Immunother 2017;66(4):475–489
Wang Y, et al. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology 2018;7(7):e1440169
Chen Y, et al. Chimeric antigen receptor-engineered T-cell therapy for liver cancer. Hepatobiliary Pancreat Dis Int 2018;17(4):301–309
Qasim W, et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol 2015;62(2):486–491
Spear TT, et al. TCR gene-modified T cells can efficiently treat established hepatitis C-associated hepatocellular carcinoma tumors. Cancer Immunol Immunother 2016;65(3):293–304
Zhu W, et al. Identification of alpha-fetoprotein-specific T-cell receptors for hepatocellular carcinoma immunotherapy. Hepatology 2018;68(2):574–589
Ning N, et al. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res 2012;72(7):1853–1864
Wang X, et al. Phase I trial of active specific immunotherapy with autologous dendritic cells pulsed with autologous irradiated tumor stem cells in hepatitis B-positive patients with hepatocellular carcinoma. J Surg Oncol 2015;111(7):862–867
Wang H, Wang J, Shi X, Ding Y. Genetically engineered bone marrow-derived mesenchymal stem cells co-expressing IFN-gamma and IL-10 inhibit hepatocellular carcinoma by modulating MAPK pathway. J BUON 2017;22(6):1517–1524
Szoor A, et al. T cell-activating mesenchymal stem cells as a biotherapeutic for HCC. Mol Ther Oncolytics 2017;6:69–79
Fu J, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007;132(7):2328–2339
Greten TF, et al. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother 2010;33(2):211–218
Beg MS, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig New Drugs 2017;35(2):180–188
Zhuang L, et al. Activity of IL-12/15/18 primed natural killer cells against hepatocellular carcinoma. Hepatol Int 2019;13(1):75–83
Sun F, Wang JZ, Luo JJ, Wang YQ, Pan Q. Exosomes in the oncobiology, diagnosis, and therapy of hepatic carcinoma: a new player of an old game. Biomed Res Int 2018;2018:2747461
Funding
This work was supported by the National Natural Science Foundation of China, Grant No. 81470837; Innovative Research Groups of the National Natural Science Foundation of China, Grant No. 81721002; Beijing Municipal & Technology Commission Grant No. Z171100001017183. All authors have no financial relationships relevant to this article to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lifeng Wang and Fu-Sheng Wang have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Wang, FS. Clinical immunology and immunotherapy for hepatocellular carcinoma: current progress and challenges. Hepatol Int 13, 521–533 (2019). https://doi.org/10.1007/s12072-019-09967-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09967-y