Abstract
Chronic hepatitis B virus (HBV) infection is a major global health burden and cure is rarely achieved by current antiviral therapies. Therefore, there is an urgent need for new therapeutic options. Immune modulation with the goal to restore dysfunctional HBV-specific immunity is an interesting target for new therapeutic strategies. Based on the current evidence on immunology in resolving and persistent HBV infection, we will review the growing field of immunotherapeutic approaches for treatment of chronic HBV infection. We will also discuss the challenge of a heterogeneous patient population and personalized treatment as a possible key factor of success.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the availability of a preventive vaccine, hepatitis B virus (HBV) infection is a major global health problem with over 250 million chronically infected individuals worldwide [69, 83]. The outcome of HBV infection is closely linked to age: 95% of infections acquired in adulthood are cleared spontaneously, while 90% of perinatally infected children develop chronic infection. Severe consequences of chronic HBV infection include liver cirrhosis and hepatocellular carcinoma [29, 65].
In the natural course of chronic HBV infection, five not necessarily sequential clinical phases with different underlying immunological activities can be defined [22]. They are illustrated in Fig. 1. Recently, transcriptome analysis of liver biopsies revealed distinct microRNA signatures in different phases of chronic HBV infection providing a permissive environment for viral replication [73].

(modified from [63])
Clinical phases of HBV infection
-
HBeAg-positive chronic HBV infection is characterized by high viral load, the presence of HBeAg in the serum and persistently normal serum liver enzymes (e.g., alanine aminotransferase, ALT). This phase is common in young, perinatally infected patients and has been described as a state of “immunotolerance”. In the liver, this phase is correlated with low levels of inflammation and fibrosis but ongoing integration of HBV DNA into the hepatocyte genome. The phase is associated with preserved HBV-specific T-cell function [36].
-
HBeAg-positive chronic hepatitis is characterized by the presence of serum HBeAg and an increase in serum liver enzymes. In the liver, this phase is correlated with increasing inflammation and progression to fibrosis that is most likely mediated by increased intrahepatic immunity.
-
HBeAg-negative chronic HBV infection, which has been previously known as “asymptomatic HBsAg carrier status”, is thought to be a state of immune control of infection. It is characterized by the presence of serum antibodies to HBeAg (anti-HBe), low viral load and serum liver enzymes persistently within the normal range. In the liver, this phase is correlated with absent or minimal inflammation und fibrosis.
-
HBeAg-negative chronic hepatitis is characterized by the absence of serum HBeAg (usually with detectable anti-HBe) and fluctuating levels of serum liver enzymes (e.g., ALT) and viral load. In the liver, this phase is associated with high levels of inflammation and fibrosis.
-
HBsAg-negative phase is characterized by negative serum HBsAg with or without detectable antibodies to HBsAg (anti-HBs) and usually undetectable HBV DNA in the serum. This phase may be reached in the course of spontaneous viral clearance in a minority of patients and still bears risk of HBV reactivation under immunosuppressive conditions.
Therapeutic options for patients with chronic HBV infection are currently limited. Treatment with pegylated interferon achieves viral clearance in about 10–20% of Caucasian and < 5% of Asian patients [14] while side effects are significant. Treatment with nucleotide or nucleoside analogues (NA) leads to suppression of viral DNA and normalization of liver enzymes, while lifelong treatment is mostly required as HBsAg loss is rarely achieved. Therefore, there is an urgent need for new therapeutic approaches.
How is HBV “cure” defined?
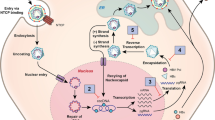
While highly effective treatment options for other hepatotropic viruses such as hepatitis C virus (HCV) have emerged recently, HBV therapy remains challenging. This is partly due to the viral replication strategy of HBV. After infection of hepatocytes through the sodium taurocholate cotransporting polypeptide (NTCP), viral DNA is transported to the nucleus and converted into a minichromosome (covalently closed circular DNA (cccDNA)) to serve as a template for viral transcription. Some parts of the viral DNA are even integrated into the genomic hepatocyte DNA. cccDNA as well as integrated viral DNA persist in the nucleus and represent a reservoir for reactivation even after spontaneous viral clearance [53]. Elimination of cccDNA and integrated viral DNA remain challenging and raise the question of the appropriate definition of HBV cure. Recently, consensus definitions have been proposed as treatment endpoints of new HBV therapies [44] illustrated in Fig. 2.
Different definitions of HBV cure as proposed by Lok et al. [44]
-
“Partial cure” describes a state of persistent chronic infection with normalization of serum liver enzymes and low viral load. It resembles the phase of HBeAg-negative chronic HBV infection and is associated with a reduced risk of severe liver disease and hepatocellular carcinoma. Partial cure is the goal of current NA therapy.
-
“Functional cure” is defined as a state of HBsAg loss and undetectable HBV DNA in the serum after a definitive treatment, resembling the state of spontaneous viral clearance after acute infection. It has been proposed as a reasonable goal for future clinical trials. The significance of seroconversion to anti-HBs still needs to be determined. While there is evidence for an equal sustainability of HBsAg loss and seroconversion to anti-HBs [71, 89], the risk of HBV reactivation seems to be increased for anti-HBs-negative patients [70].
-
“Complete cure” has been described as an additional complete eradication of HBV cccDNA from hepatocytes.
-
“Sterilizing cure” was attributed to a situation of complete eradication of HBV DNA from the liver (including integrated genomic DNA), with subsequential absence of the risk for HBV reactivation.
Lessons from natural cure
The majority of HBV infections acquired in adulthood are cleared spontaneously. This points towards an important role of the host immune responses for control of HBV infection. Development of chronic HBV infection is associated with failure of HBV-specific immune responses. Therefore, immune modulation with the goal to restore dysfunctional HBV-specific immunity is an interesting target for new therapeutic strategies. The key players responsible for natural cure will be summarized in the following with a main focus on adaptive immunity.
Innate immunity
There is an ongoing debate regarding the importance of innate immunity for the natural cure of HBV infection. Evidence supporting the thesis of HBV as a “stealth virus” and evidence showing activation of innate immunity are discussed in detail in the section “failure of natural cure”. There is growing evidence for an important role of different innate immune cells such as monocytes, NK cells, non-classical T cells and immature myeloid-derived cells regarding control of HBV infection but also regulation of immune action. Their function and biochemical relevance have recently been reviewed elsewhere [46]. Activation of NK cells has been demonstrated in patients with subclinical and acute HBV infection [20, 24]. Recently, activation of a subset of cytolytic NK cells with intermediate CD56 expression was found to be associated with early HBsAg clearance [90].
CD8+ T cells
It is generally accepted that the adaptive immune response is required for HBV clearance and control. Indeed, the importance of CD8+ T cells for HBV clearance has been demonstrated by depletion studies in chimpanzees [75]. In patients, spontaneous viral clearance is associated with vigorous, multispecific CD8+ T-cell responses [48]. Longitudinal analysis of HBV-specific CD8+ T cells in patients with acute HBV infection revealed the emergence of CD127+ cells with a functional and phenotypical profile of antigen-specific CD8+ memory T cells upon spontaneous clearance of HBV infection [9]. Antiviral CD8+ T-cell activity is mediated via cytolytic and non-cytolytic effector functions. Killing of hepatocytes by FasL/Fas-interaction and secretion of perforin and granzymes have early been identified to be mediators of liver damage during acute hepatitis [4]. More recent data from the mouse model revealed a complex interplay and recruitment of antigen-nonspecific cells and platelets to the liver, promoting cytolytic activity of CD8+ T cells [32]. In co-culture experiments of HBV-specific CD8+ T cells with HBV-infected HepG2 cells, direct cell contact and killing of hepatocytes were associated with the strongest decrease in HBV DNA compared to non-cytolytic pathways [30]. However, in chimpanzees with acute, spontaneously resolving HBV infection, the HBV DNA in serum and liver decreased weeks before the peak of liver damage underlining the importance of non-cytolytic antiviral pathways [27, 28]. Non-cytolytic CD8+ T-cell effector functions include the secretion of IFN-γ and TNF-α and are able to clear cccDNA from hepatocytes [86].
CD4+ T cells
CD4+ T cells also play an important but rather indirect role in viral clearance during acute HBV infection. Indeed, in the chimpanzee model, it has been shown that the depletion of CD4+ T cells 6 weeks after infection did not impair HBV clearance [75], while CD4+ T-cell depletion before infection leads to viral persistence most likely due to impaired induction of a virus-specific CD8+ T-cell response [3]. In patients, vigorous, multispecific HBV-specific CD4+ T-cell responses are correlated with viral clearance. A possible mechanism shaping the CD4+ T-cell repertoire is genetic variability of HLA class II alleles involved in CD4+ T-cell antigen presentation, with some alleles showing an association with spontaneous clearance of HBV infection [19, 72]. Of note, in mice CD4+ follicular helper T-cell responses were also shown to be required for viral clearance [81].
B cells
A central role of B cells in HBV prevention has long been proven by the success of the recombinant HBsAg vaccine inducing seroprotection in > 96% of vaccinated persons [77]. In line with this, early data described the presence of antibodies reacting with Dane particles in patients with spontaneous resolving HBV infection, while these antibodies were absent in patients with chronic HBV infection [2]. The importance of the humoral immune response for spontaneous clearance of HBV infection is further supported by the observation of HBV reactivation after B-cell depleting therapy with the recombinant CD20 antibody rituximab [23]. Interestingly, the risk of HBV reactivation in patients with resolved HBV infection is reduced by the presence of anti-HBs antibody compared to patients with antibody to hepatitis B core antigen only [58]. This finding suggests a possible difference regarding protective capacity for different antibody specificities in HBV infection and points towards an important role of anti-HBsAg for protection against HBV reactivation.
Failure of natural cure
About 90% of HBV infections acquired perinatally and 5% of infections acquired in adulthood fail to be cleared spontaneously and persist instead [29]. Reasons for HBV persistence include host and viral factors. Of particular importance is the failure of HBV-specific immune responses.
Innate immunity
The role of innate immunity for chronic HBV infection is not well defined. HBV has been proposed to be a “stealth virus” [13] that does not cause the induction of an innate immune response. In support of this, a type I IFN response was not induced immediately after HBV infection in chimpanzees [85]. The exact mechanisms of this “invisibility” of HBV to the innate immune system remain elusive and some research groups even suggested an active suppression of innate immunity by HBV proteins. Recently, data from cell culture and mouse model showed downregulation of interferon-stimulated genes by epigenetic modification through HBx protein as a possible mechanism [41]. Other groups argue that HBV evades interferon induction as well as interferon-induced antiviral effects. A possible mechanism might be escape from DNA-sensing mediated by cyclic guanosine monophosphate–adenosine monophosphate synthase (cGAS) and the downstream adaptor protein stimulator of IFN genes (STING). Human and murine hepatocytes have been found to lack expression of STING possibly facilitating chronic HBV infection [76]. Recently, also inhibition of cGAS expression by HBV has been suggested as a potential evasion mechanism from innate immunity [79]. In HCV-infected cells, however, HBV coinfection did not inhibit the interferon-mediated suppression of HCV replication pointing towards evasion of innate immunity rather than active suppression by HBV [52]. In contrast, data mainly from cell culture and mouse models demonstrate a possible activation of an innate immunity, for example by RIG-I sensing and induction of type III interferons [66]. In line with this, the group of Protzer argued that early recognition of HBV infection is conducted by liver macrophages (Kupffer cells) and Interleukin-6 rather than interferon [31].
Studies on NK cells provide conflicting results with some studies claiming defective, others an enhanced NK-cell function in patients with chronic HBV infection. Non-cytolytic antiviral effector functions of NK cells seem to be impaired in chronic HBV infection [54]. At the same time, HBV-related liver damage seems to be exacerbated by enhanced cytolytic NK-cell-mediated effector functions [78]. Possibly, NK-cell functions might be influenced differentially by shifts of their complex inhibitory and activating receptors and by a different cytokine milieu. This may also explain the various reported antiviral and regulatory NK-cell functions (see review [46]).
CD8+ T cells
A hallmark of chronic HBV infection is CD8+ T-cell failure that is most likely caused by two main mechanisms: the emergence of viral escape mutations and CD8+ T-cell exhaustion. The role of viral escape in the context of HBV infection is controversial. Early studies suggested that viral escape is not common for a set of well-described HLA-A2-restricted epitopes [62]. More recently, a study using a viral sequencing approach has found HLA-dependent sequence variations as evidence for T-cell selection pressure [18]. Subsequent functional analysis of the HLA-dependent sequence variations confirmed the proposed CD8+ T-cell epitopes and demonstrated impaired recognition of the selected variants by HBV-specific CD8+ T cells [35]. A longitudinal analysis showing the appearance of escape mutations in the course of HBV infection, however, is still missing.
CD8+ T-cell exhaustion is a state of impaired effector functions and reduced proliferative capacity that is characterized by the expression of inhibitory molecules (e.g., PD-1, CTLA-4, Tim3, Lag3, TIGIT) on the cell surface [10]. Driving factors of CD8+ T-cell exhaustion include high antigen exposure [82], lack of T-cell help and the immunosuppressive liver environment characterized by the action of immunosuppressive cytokines (IL-10, TGF-β) mainly produced by regulatory T cells, B cells and stellate cells and the release of enzymes like arginase by damaged hepatocytes [6]. In the mouse model of LCMV, different subsets of exhausted CD8+ T cells characterized by distinct expression levels of surface receptors (PD-1) and transcription factors (T-bet, Eomesodermin/Eomes) have been found to represent different phases of exhaustion [56]. Terminally exhausted LCMV-specific CD8+ T cells were characterized by a high surface expression of PD-1, low expression of T-bet and high expression of Eomes. Less exhausted LCMV-specific CD8+ T cells with a partially retained potential of antiviral effector functions were characterized by an intermediate PD-1 expression, a high expression of T-bet and a low expression of Eomes. The different subsets have been found to be differentially responsive to blockade by inhibitory receptors [33]. While terminally exhausted CD8+ T cells were unresponsive to PD-1 blockade, less exhausted CD8+ T cells could be rescued by PD-1 blockade (for an overview see review [84]). Reasons for the lack of full recovery include epigenetic alterations in exhausted CD8+ T cells that are only partially reversible by PD-1 blockade [57]. In patients, a detailed analysis of subsets of exhausted HBV-specific CD8+ T cells is not yet available. However, in vitro experiments found a link between stage of CD8+ T-cell differentiation and response to PD-1 blockade in patients with chronic HBV infection [5]. Transcriptome analysis of exhausted CD8+ T cells in patients with chronic HBV infection has also revealed substantial downregulation of mitochondrial function and cell metabolism [26]. Exhausted HBV-specific CD8+ T cells seem to be dependent on glycolysis and mitochondrial defects may limit their capacity to exploit alternative metabolic pathways [68].
CD4+ T cells
While the concept of CD8+ T-cell failure is well established, little is known about CD4+ T-cell function during chronic HBV infection. A study using a new HBV-specific MHC class II tetramer has shown weak CD4+ T-cell responses in patients with chronic HBV infection compared to patients with acute HBV infection. The HBV-specific CD4+ T cells present during chronic HBV infection showed a dysfunctional phenotype characterized by upregulation of PD-1, while other coinhibitory receptors (CTLA-4, TIM-3, KLRG1 and CD244) were not significantly upregulated [61]. Recently, an impaired CD4+ follicular helper T-cell (Tfh cell) response against HBsAg has been reported in chronic HBV-infected patients [81]. Meanwhile, a subset of Treg-like Tfh cells was enriched and co-culture experiments suggested that this subset might mediate differentiation of immature IL-10 producing “regulatory B cells (Breg)” [80].
B cells
There is growing evidence that B-cell function might be impaired during chronic HBV infection. Early data found a reduced frequency of HBsAg-specific B cells in peripheral blood samples of patients with chronic compared to acute hepatitis B infection [8]. Also, antibodies and immune complexes have been detected with a higher frequency in patients who cleared HBV infection spontaneously compared to chronic carriers [45]. More recent ex vivo analysis of B cells from the peripheral blood of patients with chronic hepatitis B infection showed an activated B-cell phenotype with defective proliferative capacity. Markers of terminal differentiation indicating exhaustion, however, were absent [55]. Data comparing B cell phenotype in different stages of infection indicate a possible negative correlation of activation markers with immune control of chronic HBV infection and spontaneous viral clearance [88].
In patients with chronic HBV infection, functional analysis revealed the presence not only of antibody-producing, but also of immature IL-10 producing “regulatory B cells (Breg)”. In vitro blockade of IL-10 and depletion of Breg enhanced full functionality of HBV-specific T cells. As supporting clinical evidence, a correlation between the frequency of Breg and hepatic flares had been reported [16]. In co-culture experiments, Breg inhibited IFN γ and IL-17 production of CD4+ T cells, while differentiation to Treg was enhanced [43].
While B cells seem to be reduced and potentially dysfunctional in the peripheral blood of chronically HBV-infected patients analysis of liver biopsies showed the presence of CD20-positive B-cell aggregates in portal areas and diffuse infiltration of single cells in the lobules. Noteworthy, the extent of infiltration correlated with stage of liver inflammation and fibrosis [51]. However, functional impact and significance of these intrahepatic B-cell aggregates require further research.
Lessons from current antiviral therapy
HBsAg loss occurs generally in about 1% of untreated chronically infected patients per year and is not common in patients undergoing NA therapy [49]. Usually, lifelong NA treatment is performed to ensure suppression of viremia, while discontinuation facilitates relapse of viremia and hepatitis. In a few patients, NA treatment discontinuation is not followed by flares and relapse illustrated in Fig. 3 [15]. Current clinical and virological parameters do not allow patient selection for NA therapy discontinuation. As immune responses are mediators of viral control, recent studies have evaluated their use as possible novel biomarkers. A longitudinal analysis of T-cell responses in patients undergoing NA therapy now identified the presence of core- and polymerase-specific CD8+ T-cell responses as a possible biomarker for the safety of NA therapy discontinuation [64]. Analysis of HBV-specific CD8+ T-cell responses in patients after long-term NA therapy showed a restoration of proliferative capacity and effector functions compared to untreated chronically HBV-infected patients [11].
Possible restoration of HBV-specific CD8+ T cell funtion and proliferative activity after long-term NA therapy [11]. PD-1 and TIM-3 are upregulated on HBV-specific CD8+ T cells after long-term NA therapy; core- and pol-specific T cell responses might represent novel biomarkers for the safety of treatment discontinuation after long-term NA therapy [64]. CTLA-4 cytotoxic T-lymphocyte-associated protein 4, LAG-3 lymphocyte-activation gene 3, TIM-3 T cell immunoglobulin and mucin domain 3, PD-1 programmend death receptor 1, TNF-alpha tumor necrosis factor alpha, IFN-gamma interferon gamma, IL-2 interleukin-2, TCR T cell receptor, MHC class I major histocompatibility complex class I, core HBV core protein, Pol HBV polymerase protein
Interferon treatment achieves viral clearance in about 10–20% of Caucasian and < 5% of Asian patients, sometimes years after treatment administration, while side- effects are significant [14]. There is evidence for both direct antiviral and immunomodulatory effects of IFN-α therapy, while the differential responsiveness of patients and the role of different molecular pathways is not well understood (see also [74]).
Immunotherapeutic perspectives
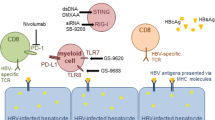
There is a broad field of new immunotherapeutic approaches and some have already shown promising results in animal models and early clinical trials. These approaches have different objectives (Fig. 4): (1) to restore dysfunctional HBV-specific immune responses (e.g., TLR7 agonist and checkpoint inhibitors); (2) to induce novel HBV-specific immune responses (e.g., therapeutic vaccination); (3) to replace host T-cell responses (e.g., T-cell engineering).
One promising immunotherapeutic candidate is pathogen-associated molecular pattern receptor TLR7 agonist vesatolimod (GS-9620). Indeed, treatment with vesatolimod led to a rapid virological response, clear reduction of risk for hepatocellular carcinoma and HBsAg loss in the woodchuck model of chronic viral hepatitis upon short-term administration [50]. In chimpanzees, treatment with vesatolimod resulted in a sustained suppression of HBV DNA, induced interferon-stimulated genes and stimulated activity of NK cells and lymphocytes [38]. The sequential analysis of liver biopsies from chronically HBV-infected chimpanzees treated with TLR7 agonist revealed the presence of intrahepatic lymphoid structures consisting of B cells and T cells at the time of treatment response [40]. Further research is needed to clarify the relative contribution of different lymphocyte populations and molecular mechanisms of B cell–T cell communication in this setting. In patients, early clinical trials have demonstrated safety of vesatolimod administration in humans. While the treatment with low doses showed no significant effect on viral DNA or serum HBsAg levels, the induction of T-cell and NK-cell responses as well as induction of interferon-stimulated genes was demonstrated [1, 12].
The concept of CD8+ T-cell exhaustion as an important mechanism of T-cell failure and viral persistence provides the rationale for the use of checkpoint inhibitors in HBV therapy. The most prominent exhaustion marker in the context of HBV infection is PD-1. Comparative analysis in vitro has shown a dominance of PD-1 expression on HBV-specific CD8+ T cells from patients with chronic HBV infection and the strongest increase in functionality upon blockade compared to other inhibitory receptors [5]. In the woodchuck model of chronic viral hepatitis, PD-1 blockade in combination with entecavir and therapeutic vaccination led to virological response and seroconversion in 2 out of 3 woodchucks [42]. In patients with advanced hepatocellular carcinoma and HBeAg-negative chronic HBV infection, however, a phase 1/2 clinical trial showed limited antiviral activity of PD-1 blockade [21]. Recently, a phase 1 clinical trial of nivolumab in patients with HBeAg-negative chronic HBV infection (n = 20) demonstrated safety and modest decline of viral DNA; one patient showed seroconversion (Data presented at EASL 2017, http://www.natap.org/2017/EASL/EASL_55.htm). Other co-inhibitory molecules present on exhausted HBV-specific CD8+ T cells in chronic HBV infection include CTLA-4, CD244/2B4, Tim-3 and LAG-3 (Table 1). In vitro studies have shown a restoration of function and proliferative capacity of peripheral blood and intrahepatic CD8+ T cells by blockade of CTLA-4 and CD244/2B4 [5, 60, 67]. Case reports suggest a possible effect of CTLA-4 blockade with ipilimumab on HBV infection in patients with advanced melanoma [59]. Interestingly, checkpoint inhibitors do not only affect CD8+ T cells but also influence other immune cells. Recently, in an HBV mouse model, CTLA-4 blockade has been shown to block activity of regulatory T cells while activity of CD4+ Tfh cells was enhanced [81]. Further research will need to clarify the effect and possible therapeutic use of checkpoint inhibition in patients with chronic HBV infection.
A further immunotherapeutic approach is the induction of novel immune responses by therapeutic vaccination. While preclinical data showed promising results, clinical trials using HBsAg-based prophylactic vaccines failed to show lasting therapeutic effects [39, 87]. Currently, there is an increasing interest in the development of vaccines based on other hepatitis B viral antigens or the combination and sequential use of NA therapy with therapeutic vaccination and other immunotherapeutic strategies [17].
While the majority of immunotherapeutic concepts aim to reinforce the dysfunctional HBV-specific immune response of the host, T-cell engineering tries to artificially replace exhausted HBV-specific CD8+ T cells. Recently, data from the mouse model showed a significant antiviral effect of engineered CD8+ T cells transiently expressing HBV-specific T-cell receptors [34]. The group of Bertoletti was also able to produce “resting” HBV-specific T cells that limited infection of HBV-infected human hepatocytes in mice mainly by non-cytolytic pathways [37]. Obstacles for a possible future clinical use of engineered T cells include the complex and costly production as well as strict regulations concerning the use of genetically modified cells in humans. A comprehensive overview on T-cell therapy for chronic HBV infection is provided by Bertoletti et al [7].
Challenges
Although several new immunotherapeutic candidates are in the pipeline, the current state of virological and immunological evidence suggests that HBV cure by one single substance will be difficult to achieve. Instead, a combination of different approaches and personalized treatment might be a key factor of success [47].
Data from the mouse model of LCMV showing distinct stages of T-cell exhaustion with differential restoration capacity implicate that immune therapy in chronic infection might have to be individually tailored according to CD8+ T-cell phenotype in different phases of infection (see section “failure of natural cure”/”CD8+ T cells”). In line with that, in vitro experiments showed a link between stage of HBV-specific CD8+ T-cell differentiation and response to PD-1 blockade in patients with chronic HBV infection [5]. The exact characterization of CD8+ T-cell subpopulations in patients in different stages of HBV infection and their responsiveness to checkpoint inhibitors have yet to be performed. Noteworthy, one study analyzing CD8+ T-cell responses in the “immunotolerant” HBeAg-positive chronic HBV infection of young patients found a less exhausted CD8+ T-cell phenotype compared to other stages of chronic HBV infection occurring later in life [36]. This study challenges the concept of “immunotolerance” but rather suggests that immunotherapy should be considered early in life. In support of this, recent transcriptome analysis in untreated patients with chronic HBV infection revealed distinct blood gene signatures during different phases of chronic HBV infection suggesting differential activation of B cells, T cells and NK cells. Immunoactivation of innate interferon and B-cell signatures during HBeAg-positive chronic HBV infection again challenges the concept of “immunotolerance” [78]. Figure 5 summarizes possible concepts for the use of immunotherapy adapted to immunological phases of chronic HBV infection based on the limited evidence available to date. During all phases of chronic HBV infection with high antigen load, NA therapy that may lead to restoration of HBV-specific CD8+ T cells should be considered prior to immunotherapy. In HBeAg-positive chronic HBV infection, a low level of liver inflammation and partially preserved T-cell function might favor the use of either immunotherapeutic approaches that should restore the HBV-specific immune responses (e.g., checkpoint inhibitors, TLR agonist) or approaches that induce new HBV-specific immune responses (e.g., therapeutic vaccination). In patients with HBeAg-positive chronic hepatitis with increased liver inflammation and higher level of CD8+ T-cell exhaustion, immunotherapies that restore the HBV-specific immune responses (e.g., checkpoint inhibitors, TLR agonist) might be considered. The phase of HBeAg-negative chronic infection may be treated similarly to HBeAg-positive chronic infection with low level of liver inflammation and partially preserved T-cell function. During HBeAg-negative chronic hepatitis, high levels of liver inflammation and CD8+ T-cell exhaustion may favor the use of immunotherapeutic approaches that replace dysfunctional HBV-specific immune responses (e.g., T-cell engineering). The HBsAg-negative phase resembles functional cure and usually does not require treatment.
Possible therapeutic concepts adapted to immunological phases of chronic HBV infection based on the limited evidence available to date. HBeAg hepatitis B e antigen, Anti-HBe antibody to hepatitis B e antigen, HBsAg hepatitis B surface antigen, Anti-HBs antibody to Hepatitis B surface antigen, ALT alanine aminotransferase, NA nucleotide/nucleoside analogues, TLR toll like receptor
While immunotherapy provides promising approaches for the cure of HBV infection, future research needs to clarify also possible risks and side effects. A major concern is a possible induction of autoimmunity or overwhelming immune responses.
References
Agarwal K, Ahn SH, Elkhashab M, Lau AH, Gaggar A, Bulusu A, et al. Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J Viral Hepat 2018;25:1331–1340
Alberti A, Diana S, Sculard GH, Eddleston AL, Williams R. Detection of a new antibody system reacting with Dane particles in hepatitis B virus infection. Br Med J 1978;2:1056–1058
Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, et al. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol 2009;83:9652–9662
Balkow S, Kersten A, Tran TTT, Stehle T, Grosse P, Museteanu C, et al. Concerted action of the FasL/Fas and perforin/granzyme A and B pathways is mandatory for the development of early viral hepatitis but not for recovery from viral infection. J Virol 2001;75:8781–6791
Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol 2014;61:1212–1219.
Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol 2016;64:S71–S83
Bertoletti A, Tan AT, Koh S. T-cell therapy for chronic viral hepatitis. Cytotherapy 2017;19:1317–1324
Meyer Zum Büschenfelde KH, Löhr HF. Regulation of the neutralizing anti-hepatitis B surface (HBs) antibody response in vitro in HBs vaccine recipients and patients with acute or chronic hepatitis B virus (HBV) infection. Clin Exp Immunol 1996;105:52–58
Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, et al. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol 2006;80:3532–3540
Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007;81:4215–4225
Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012;143:963.e9–973.e9
Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, et al. TLR7 agonist increases responses of hepatitis B virus-specific T cells and natural killer cells in patients with chronic hepatitis B treated with nucleos(t)ide analogues. Gastroenterology 2018;154:1764.e7–1777.e7
Cheng X, Xia Y, Serti E, Block PD, Chung M, Chayama K, et al. Hepatitis B virus evades innate immunity of hepatocytes but activates cytokine production by macrophages. Hepatol Baltim Md 2017;66:1779–1793.
Chu C-M, Liaw Y-F. Hepatitis B surface antigen seroclearance during chronic HBV infection. Antivir Ther 2010;15:133–143
Cornberg M, Höner Zu Siederdissen C. HBsAg seroclearance with NUCs: rare but important. Gut 2014;63:1208–1209.
Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, et al. IL-10–producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol 2012;189:3925–3935
Dembek C, Protzer U, Roggendorf M. Overcoming immune tolerance in chronic hepatitis B by therapeutic vaccination. Curr Opin Virol 2018;30:58–67
Desmond CP, Gaudieri S, James IR, Pfafferott K, Chopra A, Lau GK, et al. Viral adaptation to host immune responses occurs in chronic hepatitis B virus (HBV) infection, and adaptation is greatest in HBV e antigen-negative disease. J Virol 2012;86:1181–1192.
Diepolder HM, Jung MC, Keller E, Schraut W, Gerlach JT, Grüner N, et al. A vigorous virus-specific CD4+ T cell response may contribute to the association of HLA-DR13 with viral clearance in hepatitis B. Clin Exp Immunol 1998;113:244–251
Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology 2009;137:1289–1300
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–2502
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, and European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–98
Evens AM, Jovanovic BD, Su Y-C, Raisch DW, Ganger D, Belknap SM, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol 2011;22:1170–1180
Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A, et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 2009;58:974–982
Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 2010;138(2):682–693.e4
Fisicaro P, Barili V, Montanini B, Acerbi G, Ferracin M, Guerrieri F, et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat Med 2017;23:327–336
Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol 2006;1:23–61
Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science 1999;284:825–829
Hadziyannis SJ. Natural history of chronic hepatitis B in Euro-Mediterranean and African countries. J Hepatol 2011;55:183–191
Hoh A, Heeg M, Ni Y, Schuch A, Binder B, Hennecke N, et al. Hepatitis B virus-infected HepG2hNTCP cells serve as a novel immunological tool to analyze the antiviral efficacy of CD8+ T cells in vitro. J Virol 2015;89:7433–7438
Hösel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatol Baltim Md 2009;50:1773–1782
Iannacone M, Sitia G, Ruggeri ZM, Guidotti LG. HBV pathogenesis in animal models: recent advances on the role of platelets. J Hepatol 2007;46:719–726
Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016;537:417–421
Kah J, Koh S, Volz T, Ceccarello E, Allweiss L, Lütgehetmann M, et al. Lymphocytes transiently expressing virus-specific T cell receptors reduce hepatitis B virus infection. J Clin Investig 2017;127:3177–3188
Kefalakes H, Budeus B, Walker A, Jochum C, Hilgard G, Heinold A, et al. Adaptation of the hepatitis B virus core protein to CD8(+) T-cell selection pressure. Hepatol Baltim Md 2015;62:47–56
Kennedy PTF, Sandalova E, Jo J, Gill U, Ushiro-Lumb I, Tan AT, et al. Preserved T-cell function in children and young adults with immune-tolerant chronic hepatitis B. Gastroenterology 2012;143:637–645
Koh S, Kah J, Tham CYL, Yang N, Ceccarello E, Chia A, et al. Nonlytic lymphocytes engineered to express virus-specific T-cell receptors limit HBV infection by activating APOBEC3. Gastroenterology 2018;155:180–193.e6
Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013;144(1508–1517):1517.e1–1517.e10
Lee YB, Lee J-H, Kim YJ, Yoon J-H, Lee H-S. The effect of therapeutic vaccination for the treatment of chronic hepatitis B virus infection. J Med Virol 2015;87:575–582
Li L, Barry V, Daffis S, Niu C, Huntzicker E, French DM, et al. Anti-HBV response to toll-like receptor 7 agonist GS-9620 is associated with intrahepatic aggregates of T cells and B cells. J Hepatol 2018;68:912–921
Lim K-H, Park E-S, Kim DH, Cho KC, Kim KP, Park YK, et al. Suppression of interferon-mediated anti-HBV response by single CpG methylation in the 5’-UTR of TRIM22. Gut 2018;67:166–178
Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog 2014;10:e1003856
Liu Y, Cheng L-S, Wu S, Wang S-Q, Li L, She W-M, et al. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection. Clin Sci Lond Engl 2016;1979(130):907–919
Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: from discovery to regulatory approval. J Hepatol 2017;67:847–861
Madaliński K, Bragiel I. HBsAg immune complexes in the course of infection with hepatitis B virus. Clin Exp Immunol 1979;36:371–378
Maini MK, Gehring AJ. The role of innate immunity in the immunopathology and treatment of HBV infection. J Hepatol 2016;64:S60–S70
Maini MK, Pallett LJ. Defective T-cell immunity in hepatitis B virus infection: why therapeutic vaccination needs a helping hand. Lancet Gastroenterol Hepatol 2018;3:192–202
Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, et al. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology 1999;117:1386–1396
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013;381:468–475
Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol 2015;62:1237–1245
Mohamadkhani A, Naderi E, Sotoudeh M, Katoonizadeh A, Montazeri G, Poustchi H. Clinical feature of intrahepatic B-lymphocytes in chronic hepatitis B. Int J Inflamm 2014;2014:896864
Mutz P, Metz P, Lempp FA, Bender S, Qu B, Schöneweis K, et al. HBV bypasses the innate immune response and does not protect HCV from antiviral activity of interferon. Gastroenterology 2018;154:1791.e22–1804.e22
Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015;64:1972–1984
Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 2009;137(1151–1160):1160.e1–1160.e7
Oliviero B, Cerino A, Varchetta S, Paudice E, Pai S, Ludovisi S, et al. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol 2011;55:53–60
Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012;338:1220–1225
Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016;354:1160–1165
Paul S, Dickstein A, Saxena A, Terrin N, Viveiros K, Balk EM, et al. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: a meta-analysis. Hepatol Baltim Md 2017;66:379–388
Ravi S, Spencer K, Ruisi M, Ibrahim N, Luke JJ, Thompson JA, et al. Ipilimumab administration for advanced melanoma in patients with pre-existing Hepatitis B or C infection: a multicenter, retrospective case series. J Immunother Cancer 2014;2:33
Raziorrouh B, Schraut W, Gerlach T, Nowack D, Grüner NH, Ulsenheimer A, et al. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatol Baltim Md 2010;52:1934–1947
Raziorrouh B, Heeg M, Kurktschiev P, Schraut W, Zachoval R, Wendtner C, et al. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One 2014;9:e105703
Rehermann B, Pasquinelli C, Mosier SM, Chisari FV. Hepatitis B virus (HBV) sequence variation of cytotoxic T lymphocyte epitopes is not common in patients with chronic HBV infection. J Clin Investig 1995;96:1527–1534
Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005;5(3):215–229
Rivino L, Bert NL, Gill US, Kunasegaran K, Cheng Y, Tan DZM, et al. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Investig 2018;128:668–681
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, Chen DS, Chen HL, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98
Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity 2015;42:123–132
Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatol Baltim Md 2011;53:1494–1503
Schurich A, Pallett LJ, Jajbhay D, Wijngaarden J, Otano I, Gill US, et al. Distinct metabolic requirements of exhausted and functional virus-specific CD8 T cells in the same host. Cell Rep 2016;16:1243–1252
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet Lond Engl 2015;386:1546–1555
Seto W-K, Chan TSY, Hwang Y-Y, Wong DK-H, Fung J, Liu KS-H, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol 2014;32:3736–3743
Seto W-K, Cheung K-S, Wong DK-H, Huang F-Y, Fung J, Liu KS-H, Let al. Hepatitis B surface antigen seroclearance during nucleoside analogue therapy: surface antigen kinetics, outcomes, and durability. J Gastroenterol 2016;51:487–495
Shin E-C, Sung PS, Park S-H. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol 2016;16:509–523
Singh AK, Rooge SB, Varshney A, Vasudevan M, Bhardwaj A, Venugopal SK, et al. Global microRNA expression profiling in the liver biopsies of hepatitis B virus-infected patients suggests specific microRNA signatures for viral persistence and hepatocellular injury. Hepatol Baltim Md 2018;67:1695–709
Thimme R, Dandri M. Dissecting the divergent effects of interferon-alpha on immune cells: time to rethink combination therapy in chronic hepatitis B? J Hepatol 2013;58:205–209
Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003;77:68–76
Thomsen MK, Nandakumar R, Stadler D, Malo A, Valls RM, Wang F, et al. Lack of immunological DNA sensing in hepatocytes facilitates hepatitis B virus infection. Hepatol Baltim Md 2016;64:746–759
van den Ende C, Marano C, van Ahee A, Bunge EM, De Moerlooze L. The immunogenicity of GSK’s recombinant hepatitis B vaccine in children: a systematic review of 30 years of experience. Expert Rev Vaccines 2017;16:789–809
Vanwolleghem T, Hou J, van Oord G, Andeweg AC, Osterhaus ADME, Pas SD, et al. Re-evaluation of hepatitis B virus clinical phases by systems biology identifies unappreciated roles for the innate immune response and B cells. Hepatol Baltim Md 2015;62:87–100
Verrier ER, Yim S-A, Heydmann L, El Saghire H, Bach C, Turon-Lagot V, et al. Hepatitis B virus evasion from cyclic guanosine monophosphate-adenosine monophosphate synthase sensing in human hepatocytes. Md: Hepatol Baltim; 2018
Wang R, Xie R, Song Z. Circulating regulatory Tfh cells are enriched in patients with chronic hepatitis B infection and induce the differentiation of regulatory B cells. Exp Cell Res 2018;365:171–176
Wang X, Dong Q, Li Q, Li Y, Zhao D, Sun J, et al. Dysregulated response of follicular helper T cells to hepatitis B surface antigen promotes HBV persistence in mice and associates with outcomes of patients. Gastroenterology 2018;154:2222–2236
Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492–499
WHO, W.H. Global hepatitis report 2017. Geneva: World Health Organization; 2017
Wieland D, Hofmann M, Thimme R. Overcoming CD8+ T-cell exhaustion in viral hepatitis: lessons from the mouse model and clinical perspectives. Dig Dis Basel Switz 2017;35:334–338
Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA 2004;101:6669–6674
Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, et al. Interferon-γ and tumor necrosis factor-α produced by t cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology 2016;150:194–205
Xu D-Z, Wang X-Y, Shen X-L, Gong G-Z, Ren H, Guo L-M, et al. Results of a phase III clinical trial with an HBsAg–HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol 2013;59:450–456
Xu X, Shang Q, Chen X, Nie W, Zou Z, Huang A, et al. Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell Mol Immunol 2015;12:309–316.
Yip TC, Wong GL, Wong VW, Tse YK, Lui GC, Lam KL, Chan HL. Durability of hepatitis B surface antigen seroclearance in untreated and nucleos(t)ide analogue-treated patients. J Hepatol 2017. https://doi.org/10.1016/j.jhep.2017.09.018
Yu W-H, Cosgrove C, Berger CT, Cheney PC, Krykbaeva M, Kim AY, et al. ADCC-mediated CD56DIM NK cell responses are associated with early HBsAg clearance in acute HBV infection. Pathog Immun 2018;3:2–18
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical requirements
This article does not contain any studies with human or animal subjects.
Conflict of interest
Julia Lang, Christoph Neumann-Haefelin and Robert Thimme declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Lang, J., Neumann-Haefelin, C. & Thimme, R. Immunological cure of HBV infection. Hepatol Int 13, 113–124 (2019). https://doi.org/10.1007/s12072-018-9912-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-018-9912-8