Abstract
Background and aims
The renin–angiotensin system (RAS) has an important role in hepatic fibrosis and portal hypertension. RAS inhibitors are already accepted in clinical fields for antihypertensive management, but their effects on hepatic fibrosis are controversial. The aim of this study was to systematically review the effects of RAS inhibitors on hepatic fibrosis based on histological assessment.
Methods
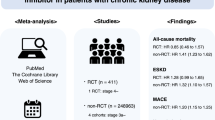
We performed a systematic review and meta-analysis (MA) of the literature using the Ovid-MEDLINE, EMBASE, and Cochrane Library databases (up to January 2015) to identify clinical studies evaluating the effects of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers on hepatic fibrosis or cirrhosis patients based on histological assessment. Of the 455 studies identified, we analyzed 7, including a total of 1066 patients, which met our selection criteria.
Results
According to the MA, patients treated with RAS inhibitors had significantly lower fibrosis scores (SMD −0.68, 95 % CI −1.03, −0.34, I 2 = 0 %, p < 0.0001) and smaller fibrosis areas (SMD −0.80, 95 % CI −1.18, −0.41, I 2 = 0 %, p < 0.0001) than controls. Serum fibrosis markers such as TGF-β1, collagen I, IV, TIMP-1, and MMP2 were significantly reduced in the intervention group. In two studies, mean arterial pressures were significantly decreased in RAS inhibitor users, but there were no reports about symptoms related to decreased blood pressure. No significant difference was found in serum creatinine levels between the intervention and control groups, and significant renal dysfunction was not observed after administration of RAS inhibitors.
Conclusions
RAS inhibitors are potential therapeutic agents for hepatic fibrosis, which can be safely used in patients with chronic liver disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Even though cirrhosis is the end stage of hepatic fibrogenesis, particularly in a compensated stage cirrhosis is currently considered potentially reversible [1–8]. Amid changing opinions, it has been suggested that the renin–angiotensin system (RAS) is an attractive antifibrotic target in the liver [1, 9, 10]. Ample evidence demonstrates that overproduction of angiotensin II during chronic liver injury promotes the activation of hepatic stellate cells, which are attributed to hepatic fibrosis [1, 10–12]. Additionally, the antifibrotic effects of angiotensin II-blocking agents have been reported in various animal models, as well as in human patients with hepatitis C virus (HCV) or alcoholic cirrhosis [1, 9–13]. In fact, angiotensin II type 1 receptor (AT1-R)-blocking agents ameliorate hepatic fibrosis in alcoholic liver disease through the inhibition of the ethanol-induced overproduction of reactive oxygen stress (ROS), which suggests that reduced oxidative stress by RAS inhibitors is associated with the prevention of hepatic fibrosis in alcoholic liver disease [13].
In response to accumulating evidence about the relationship between RAS and hepatic fibrosis, academics have begun to focus on RAS inhibitors, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) [11–13]. In light of the role that the RAS in hepatic fibrosis has demonstrated in previous literature, pharmacotherapies targeting RAS may be promising candidates for the amelioration of hepatic fibrosis [11, 14–16].
In an attempt to confirm the usefulness of RAS inhibitors for hepatic fibrosis, the current study systematically examines the histologic improvement of patients due to RAS inhibitors with a literature-based approach. Indeed, systematic review (SR) and meta-analysis (MA) have been shown to enable objective analyses of existing evidence [3–7]. Although a SR of the effects of RAS inhibitors on the reduction of portal pressure has been conducted [17], to our knowledge no SR of the effects of RAS inhibitors on hepatic fibrosis based on histological assessment currently exists. The systematic review and meta-analysis here aim to evaluate the antifibrotic effects of RAS inhibitors by focusing on studies describing the histological improvement of hepatic fibrosis based on liver biopsy [18, 19].
Methods
This study was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions and the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) group.
This review focuses on studies evaluating the effects of RAS inhibitors on hepatic fibrosis or cirrhosis patients based on histological assessment, particularly with histological hepatic fibrosis indices such as Ishak, METAVIR, or Laennec scores. Basically, we searched for studies in which intervention groups using ACE inhibitors or ARBs were compared with placebo, no-treatment, or other treatment groups. Histological changes in fibrosis were considered primary outcomes, while other hepatic fibrosis markers and side effects were considered secondary outcomes.
We conducted a SR and MA of the literature using Ovid-MEDLINE (1966 to January 2015), EMBASE (1980 to January 2015), and the Cochrane library (up to January 2015) to identify studies. The databases were searched with a combination of medical subject heading (MeSH) terms and text words for study populations and interventions, using Boolean operators. The search terms were [(liver OR hepatic) AND (cirrhosis OR fibrosis)] AND [((angiotensin-converting enzyme) OR ACE) adj inhibitor*] OR (captopril* OR cilazapril* OR enalapril* OR fosinopril* OR lisinopril* OR perindopril* OR ramipril* OR quinapril* OR teprotide) OR [(angiotensin adj3 block*) OR (candesartan OR irbesartan OR losartan OR saralasin OR telmisartan OR valsartan)]. Studies were included if they (1) were randomized or non-randomized clinical trials or observational studies, (2) described hepatic fibrosis or cirrhosis populations, (3) used ACE inhibitors or ARBs as the intervention, (4) were comparative studies between an intervention (ACE inhibitors or ARBs) and control (other treatment, placebo, etc.), and (5) had appropriate outcomes indicating the changes of hepatic fibrosis based on histological assessment. Studies were excluded if they were (1) animal studies, (2) unpublished, or (3) not published in English.
Using the search strategy described above, approximately 455 studies were identified (until January 2015). After duplicates were eliminated, 354 studies remained, 45 of which were identified as potentially relevant. Among these 45 studies, 38 were excluded for the following reasons: inappropriate study type (n = 2), no use of ACE inhibitors or ARBs as interventions (n = 1), no comparative analysis between intervention and control groups (n = 6), and no appropriate outcomes [e.g., only serum fibrosis markers or portal pressure outcomes, without histological assessment outcomes (n = 29)]. Consequently, 7 publications (with total patients n = 1066) met the selection criteria and were included in the analyses [20–26] (Fig. 1).
Data extraction and methodological quality assessment
Specific data items were extracted by two researchers (G. Kim and J. Kim) as follows: authors, study year, country, study design, duration of follow-up period, patient characteristics, sample size, type of drugs used, dose and frequency of drugs, histological differences based on histological assessment, and adverse events. Any disagreements or misunderstandings between the researchers were discussed until a consensus was reached.
The risk of bias in the selected studies was assessed using an adaptation of the Cochrane Collaboration’s tool for assessing risk of bias. The criteria involve assessing studies for publication, selection, performance, detection, attrition, and reporting bias. The methodological quality of the included studies was independently assessed by two researchers, and disagreements were presented to the whole group for resolution by consensus.
Data synthesis and analysis
Data analyses were performed using the RevMan 5.3 program from the Cochrane Collaboration to analyze the effects of RAS inhibitors. Random effects models were used, as they provide more conservative estimates in the presence of potential heterogeneity. Standardized mean differences (SMDs) were calculated as means and standard deviations (SDs) or mean change scores. Heterogeneity was assessed using the I 2 statistic. Potential publication bias was assessed by inspection of funnel plots. If significant heterogeneity was present, summary MA was abandoned, and possible sources were explored with stratified analyses. When MA was not practical, a descriptive explanation was provided.
Results
General characteristics of selected studies
Along with two randomized controlled trials (RCTs) [23, 26], one non-randomized controlled trial [25] and four cohort studies [20–22, 24] were included in the current SR and MA. Two studies were conducted in the USA [20, 21], and the other studies were conducted in Argentina [25], Canada [22], Japan [26], the Republic of Korea [23], and Spain [24]. The years of publication ranged from 2002 to 2013, and the research periods ranged on average from 6 months to approximately 7 years. While six studies [20–22, 24–26] focused on HCV, only one study [23] focused on alcoholic hepatic fibrosis. In particular, two of the six studies of HCV patients focused on liver transplantation recipients with recurrence of HCV [22, 24]. In terms of the types of intervention and control, four studies used RAS inhibitors as intervention, with other antihypertensive agents or no-treatment for hypertension as control groups [20–22, 24]. In two studies, ARBs (candesartan, losartan) with ursodeoxycholic acid (UDCA) and UDCA were used as the intervention and the control groups, respectively [23, 26]. One study compared ARB (losartan) with a no-treatment control group for hepatic fibrosis [25]. The names of the ACE inhibitors used in the selected studies were captopril, enalapril, and fosinopril, among others, and the names of the ARBs used were candesartan, irbesartan, losartan, and valsartan (Table 1).
As stated above, we collected only studies that included changes in histological scores of hepatic fibrosis. There are several hepatic fibrosis scoring systems based on histological assessment, including the Ishak score, Laennec score, METAVIR score, and so on. While the Ishak scoring system was used in three studies [20, 21, 25], the METAVIR scoring system was utilized by the other four [22–24, 26]. Among the studies using METAVIR scores, one study concurrently used the Laennec scoring system [23]. These scoring systems allow the stratification of disease severity into five to seven stages in patients with liver fibrosis or cirrhosis. The METAVIR scoring system is a five-level system, whereas the Ishak and Laennec scoring systems are seven-level systems. The Laennec scoring system, which has three different levels for cirrhosis, differs from the other scoring systems, which have only one level for cirrhosis.
Quality assessment
The majority of the included studies were judged to be at low or unclear risk of bias. In one study [23], the pathologists were blinded to the intervention, which is critical in the evaluation of hepatic fibrosis scores. The other studies [20–22, 24–26] were regarded as free of risk of bias. The intervention and control groups in all the cohort studies were comparable. In one study [20], subjects were pooled from the HALT-C trial cohort. Although the investigators did not consider the effects of the use of peg-interferon alfa-2a in the HALT-C trial, it was stated that there was no significant difference in hepatic fibrosis progression between the intervention and control groups. Therefore, we concluded that there was a low risk of selection bias in the study. The findings of another study [21] demonstrated no significant differences with regard to age, gender, ethnicity, HCV genotype, viral load, estimated duration of HCV infection, percentage of virological non-responders, alanine aminotransferase levels, and total cholesterol levels in the intervention and control groups. In light of the small possibility of selection bias in these studies [20, 21], they were considered acceptable, along with the other cohort studies [22, 24].
Histological improvement: fibrosis scores and fibrosis areas
To investigate histological improvement as a primary outcome, fibrosis scores, fibrosis progression rates, and percentages of the fibrosis area were compared. While the fibrosis scores and fibrosis progression rates were based on the hepatic fibrosis scoring systems, the fibrosis progression rate was calculated for each patient as the difference in the fibrosis scores of biopsies divided by the histological follow-up period in years, and expressed as the change in fibrosis score per year (score/year) [22, 24]. The percentages of fibrosis areas were measured in liver biopsy specimens with a computerized image analysis system [23, 26].
All seven studies reported the fibrosis scores of patients treated with RAS inhibitors [20–26]; however, from them, eight outcomes were obtained from hepatic fibrosis scores, due to the use of two scoring systems in one study [23]. In four of eight outcomes, fibrosis scores were reduced in the intervention group, whereas the scores tended to increase in the control group [21, 23–25]. On the other hand, there was no significant difference in fibrosis scores between the groups in four outcomes [20, 22, 23, 26].
Three studies were incorporated into the MA to evaluate fibrosis score improvement with the use of RAS inhibitors [23, 25, 26]. The RAS inhibitor group of the MA showed significantly lower fibrosis scores than the control group (p < 0.001) (Fig. 2a). As a result of subgroup analysis according to fibrosis scoring systems, the individual SMDs of the fibrosis scores were −1.14, −0.61, −0.70 in the Ishak, METAVIR, and Laennec scoring systems, respectively (Fig. 2a). This means that individuals in the RAS inhibitor group had lower fibrosis scores than those in control group, regardless of the scoring system.
Two studies were incorporated into the MA to evaluate fibrosis area improvement with the use of RAS inhibitors [23, 26]. The RAS inhibitor group of the MA showed significantly lower fibrosis areas than the control group (p < 0.001) (Fig. 2b). In the study by Kim et al., the percentage of the fibrosis area decreased in the intervention group (p < 0.001), whereas it increased in the control group (p = 0.308). The mean change in percentage of fibrosis areas were also significantly different between groups (p = 0.001) (Table 2) [23]. Terui et al. reported a significant decrease in the percentage of the fibrosis area in the intervention group (p < 0.001), but an increasing tendency in the control group (Table 2) [26].
Other effects: serum fibrosis markers
As secondary outcomes, serum fibrosis markers such as TGF-β1, collagen I, collagen IV, TIMP-1, MMP2, and hydroxyproline were assessed. In the studies with relevant data, TGF-β1 significantly decreased in the intervention group, but significantly increased in the control group (p < 0.05) (Table 3) [23, 26]. Other serum fibrosis markers related to gene expression in hepatic fibrosis, such as collagen I, IV, TIMP-1, and MMP2, also significantly decreased in the intervention group while they did not in control group [23, 26]. Collagen I and collagen IV were significantly decreased in each intervention groups than control group (collagen I; p < 0.001, collagen IV; p < 0.05) [26]. TIMP-1 and MMP2 also decreased significantly in the intervention group (TIMP-1; p < 0.001, MMP2; p < 0.001), whereas they increased trend in each control groups, respectively (TIMP-1; p = 0.090, MMP2; p = 0.091) (Table 3).
Lastly, the content of hepatic hydroxyproline significantly decreased in the intervention group (p < 0.001), while no significant change was seen in the control group (p = 0.162) (Table 3) [23]. Consequently, all serum fibrosis markers, including TGF-β1, collagen I, collagen IV, TIMP-1, MMP2, and hydroxyproline, decreased significantly in either group of RAS inhibitor users, which can be interpreted as an indication that RAS inhibitors might have the effect of retarding hepatic fibrosis.
Safety: blood pressure and renal function
In terms of safety issues, three studies reported relevant information about hypotension and renal dysfunction, including blood pressure and serum creatinine [23, 25, 26]. In two studies, post-mean arterial pressures were decreased in the intervention group in comparison to the control group (p < 0.05) (Table 4) [23, 26]. However, no severe decrease in mean arterial pressure was reported in any of the three studies. Furthermore, there was no report of dizziness or other symptoms related to reduced blood pressure [23]. Sookoian et al. presented information related to blood pressure, including systolic, diastolic, mean arterial pressure, and orthostatic variation in arterial pressure [25]. A non-significant change in mean arterial pressure was observed at the end of the follow-up period in comparison to baseline values. However, a loss of physiologic increase in diastolic pressure from the supine to the sitting position was observed following the intervention (p < 0.001) [25].
In relation to renal dysfunction, serum creatinine values were reported in three studies [23, 25, 26], and in none of them were there any significant difference in the serum creatinine levels of the intervention and control groups.
Discussion
There is accumulating evidence from experimental and clinical data showing that RAS is involved in liver fibrosis [1, 9–12]. Angiotensin II induces the activation of hepatic stellate cells, the main collagen-producing cells in liver, which promote hepatic fibrosis [13, 14]. Given the contributing role of angiotensin II in fibrosis, RAS inhibitors have the potential to attenuate hepatic fibrosis [15, 16].
This study is the first review to consider whether the administration of RAS inhibitors in chronic liver disease improves hepatic fibrosis, as assessed histologically. Liver biopsy is the gold standard for assessing the stage of hepatic fibrosis in patients with chronic liver disease [18, 19]. Accordingly, it is significant that fibrosis scores in this study were assessed in RAS inhibitor users with chronic liver disease who had undergone liver biopsies.
A total of eight outcomes were obtained from hepatic fibrosis scores, four of which suggested positive effects of RAS inhibitors on hepatic fibrosis [21, 23–25], whereas the remaining four demonstrated no beneficial effects of RAS inhibitors [20, 22, 23, 26]. In our review, all the relevant studies [23, 25, 26] were incorporated into the MA, regardless of the fibrosis scoring system. In MA, the RAS inhibitor group had significantly lower fibrosis scores following the intervention than the control group. In addition, a significant difference between the intervention and control groups was observed in the mean changes of fibrosis scores. One possible reason for the differences in the outcomes between the individual studies and the MA could be the small number of subjects in the individual studies, ranging from 23 to 85. In other words, retrospective studies may have less significance due to the small number of subjects.
One study showed contrasting results from different fibrosis scoring systems (METAVIR vs. Laennec scores) [23]. While a significant difference in mean changes in fibrosis scores was observed between the intervention and control groups under the Laennec scoring system (p = 0.013), no significant changes were observed under the METAVIR scoring system (p = 0.103). These contrasting results from the same subjects depending on the scoring system illustrate the need for a MA of studies using the same fibrosis scoring system. This implies that more clinical trials regarding the antifibrotic effects of RAS inhibitors in chronic liver disease patients should be conducted using histological indices.
While there were conflicting results relating to hepatic fibrosis scores from the selected studies, other indices for hepatic fibrosis, including the fibrosis area and serum markers, highlighted the positive effects of RAS inhibitors. Although histological assessment based on liver biopsy is the gold standard for the diagnosis of hepatic fibrosis, a conclusion based only on changes in fibrosis scores might not suffice. Firstly, there is the possibility of sampling error during the process of liver biopsy [27]. Secondly, changes in liver biopsy are delayed relative to changes in several serum fibrosis markers. In other words, serum fibrosis markers involved in the process of hepatic fibrosis are able to capture changes in hepatic fibrosis, which may not be captured in a liver biopsy. Therefore, the outcomes of the selected studies were comprehensively considered in our review. The results of serum fibrosis markers, including TGF-β1, collagen I, collagen IV, TIMP-1, MMP2, and hydroxyproline, suggest a positive effect of RAS inhibitors on hepatic fibrosis. Therefore, in order to confirm the antifibrotic effect, future studies that include suitable non-invasive methods such as serum fibrosis markers and Fibroscan, in addition to the liver biopsy for assessing fibrosis, would be highly desirable.
With regard to the side effects of RAS inhibitors, while significant changes in blood pressure were observed, no significant changes were observed in serum creatinine. Of the three studies showing the outcomes of mean arterial pressure analysis, two studies showed mild but significant reductions in the intervention group. In addition, although mean arterial pressure did not change significantly, a loss of physiologic increase in diastolic pressure from the supine to the sitting position was observed following the intervention. Fortunately, these three studies did not report any significant side effects related to hypotension. These results suggest that blood pressure should be carefully monitored following the administration of RAS inhibitors. Three studies reporting the effects of RAS inhibitors on serum creatinine levels demonstrated no significant changes due to the RAS inhibitors. Two studies showed no significant changes in serum creatinine levels pre- and post-administration of RAS inhibitors in intervention groups, and one study showed no difference in serum creatinine levels between the intervention and control groups. In summary, significant renal dysfunction was not observed following the administration of RAS inhibitors. However, information related to the side effects of RAS inhibitors was limited. Only three studies presented information about the impact of the treatment on the blood pressure and renal functions of patients [23, 25, 26]. Thus, more clinical trials evaluating the side effects of RAS inhibitors are required.
There are also some following potential limitations that require further discussion in the present study. First, only seven studies evaluated the effect of RAS inhibitor on hepatic fibrosis, thus limiting the robustness of the conclusions that could be reached. With regard to the design and quality of the selected studies, the four cohort studies were of relatively good quality, and were scored as acceptable (+) based on the SIGN checklist. On the other hand, one controlled trial was non-randomized and one RCT was open-label. One RCT had limited information from which to determine the quality of the study. This indicates the need for more robust study design in the evaluation of RAS inhibitors in hepatic fibrosis. Second, the characteristics of the included studies were not completely consistent, including the patient characteristics, the etiologies of cirrhosis, and the methodological difference to measure the fibrosis scoring. Third, in the present SR and MA, we only included English studies, so language bias might have influenced the results.
In conclusion, despite the discrepant results of fibrosis scores from different fibrosis scoring systems, the results of our MA indicate that the antifibrotic effects of RAS inhibitors may suggest them as a candidate of therapeutic agent for hepatic fibrosis, which can be safely used in patients with chronic liver disease. Nevertheless, future, large, multi-center, randomized controlled studies would be required to further evaluate the beneficial effect of RAS inhibitors on hepatic fibrosis shown in the present study.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ARB:
-
Angiotensin receptor blocker
- AT1-R:
-
Angiotensin II type 1 receptor
- HCV:
-
Hepatitis C virus
- MA:
-
Meta-analysis
- RAS:
-
Renin–angiotensin system
- RCT:
-
Randomized controlled trial
- ROS:
-
Reactive oxygen stress
- SMD:
-
Standardized mean difference
- SD:
-
Standard deviation
- SR:
-
Systematic review
References
Moreno M, Gonzalo T, Kok RJ, et al. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology 2010;51(3):942–952
Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med 2015;30(5):580–589
Kim G, Lee SS, Baik SK, et al. The need for histological subclassification of cirrhosis: a systematic review and meta-analysis. Liver Int 2015. doi:10.1111/liv.12923. [Epub ahead of print]
Kim G, Cho YZ, Baik SK. Assessment for risk of bias in systematic reviews and meta-analyses in the field of hepatology. Gut Liver 2015;9(6):701–706
Kim G, Eom YW, Baik SK, et al. Therapeutic effects of mesenchymal stem cells for patients with chronic liver diseases: systematic review and meta-analysis. J Korean Med Sci 2015;30(10):1405–1415
Kim G, Cho YZ, Baik SK, Kim MY, Hong WK, Kwon SO. The accuracy of ultrasonography for the evaluation of portal hypertension in patients with cirrhosis: a systematic review. Korean J Radiol 2015;16(2):314–324
Kim G, Baik SK. Overview and recent trends of systematic reviews and meta-analyses in hepatology. Clin Mol Hepatol 2014;20(2):137–150
Hong WK, Shim KY, Baik SK, et al. Relationship between tetrahydrobiopterin and portal hypertension in patients with chronic liver disease. J Korean Med Sci 2014;29(3):392–399
Kim MY, Baik SK, Park DH, et al. Angiotensin receptor blockers are superior to angiotensin-converting enzyme inhibitors in the suppression of hepatic fibrosis in a bile duct-ligated rat model. J Gastroenterol 2008;43(11):889–896
Rockey DC. Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol 2005;3(2):95–107
Pereira RM, dos Santos RA, da Costa Dias FL, Teixeira MM, Simoes e Silva AC. Renin-angiotensin system in the pathogenesis of liver fibrosis. World J Gastroenterol 2009;15(21):2579–2586
Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 2005;289(6):H2281–H2290
Kim JH, Kim JM, Cho YZ, et al. Effects of candesartan and propranolol combination therapy versus propranolol monotherapy in reducing portal hypertension. Clin Mol Hepatol 2014;20(4):376–383
Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol 2003;35(6):881–900
Munshi MK, Uddin MN, Glaser SS. The role of the renin–angiotensin system in liver fibrosis. Exp Biol Med (Maywood) 2011;236(5):557–566
Tox U, Steffen HM. Impact of inhibitors of the renin–angiotensin–aldosterone system on liver fibrosis and portal hypertension. Curr Med Chem 2006;13(30):3649–3661
Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J. Renin–angiotensin–aldosterone inhibitors in the reduction of portal pressure: a systematic review and meta-analysis. J Hepatol 2010;53(2):273–282
Kim MY, Jeong WK, Baik SK. Invasive and non-invasive diagnosis of cirrhosis and portal hypertension. World J Gastroenterol 2014;20(15):4300–4315
Mohamadnejad M, Tavangar SM, Sotoudeh M, et al. Histopathological Study of Chronic Hepatitis B: A Comparative Study of Ishak and METAVIR Scoring Systems. Int J Organ Transplant Med 2010;1(4):171–176
Abu Dayyeh BK, Yang M, Dienstag JL, Chung RT. The effects of angiotensin blocking agents on the progression of liver fibrosis in the HALT-C Trial cohort. Dig Dis Sci 2011;56(2):564–568
Corey KE, Shah N, Misdraji J, et al. The effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Liver Int 2009;29(5):748–753
Guillaud O, Gurram KC, Puglia M, et al. Angiotensin blockade does not affect fibrosis progression in recurrent hepatitis C after liver transplantation. Transplant Proc 2013;45(6):2331–2336
Kim MY, Cho MY, Baik SK, et al. Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis—a randomized open-label controlled study. Liver Int 2012;32(6):977–987
Rimola A, Londono MC, Guevara G, et al. Beneficial effect of angiotensin-blocking agents on graft fibrosis in hepatitis C recurrence after liver transplantation. Transplantation 2004;78(5):686–691
Sookoian S, Fernandez MA, Castano G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: a pilot study. World J Gastroenterol 2005;11(48):7560–7563
Terui Y, Saito T, Watanabe H, et al. Effect of angiotensin receptor antagonist on liver fibrosis in early stages of chronic hepatitis C. Hepatology 2002;36(4 Pt 1):1022
Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights 2012;7:105–117
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI15C2364), and also by the Yonsei University Future-Leading Research Initiative of 2014.
Conflict of interest
Gaeun Kim, Juyoung Kim, Yoo Li Lim, Moon Young Kim, and Soon Koo Baik declare that there are no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Kim, G., Kim, J., Lim, Y.L. et al. Renin–angiotensin system inhibitors and fibrosis in chronic liver disease: a systematic review. Hepatol Int 10, 819–828 (2016). https://doi.org/10.1007/s12072-016-9705-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-016-9705-x