Abstract
Alcoholic liver disease (ALD) is a leading cause of liver-related morbidity and mortality worldwide. ALD encompasses a spectrum of disorders including asymptomatic steatosis, steatohepatitis, fibrosis, cirrhosis and its related complications, and the acute-on-chronic state of alcoholic hepatitis. While multidisciplinary efforts continue to be aimed at curbing progression of this spectrum of disorders, there is an urgent need to focus our efforts on effective therapeutic interventions for alcoholic hepatitis (AH), the most severe form of ALD. AH is characterized by an abrupt development of jaundice and complications related to liver insufficiency and portal hypertension in patients with heavy alcohol intake. The mortality of patients with severe AH is very high (20–50 % at 3 months). The current therapeutic regimens are limited. The development of new therapies requires translational studies in human samples and suitable animal models that reproduce clinical and histological features of human AH. This review article summarizes the clinical syndrome, pre-clinical translational tools, and pathogenesis of AH at a molecular and cellular level, with the aim of identifying new targets of potential therapeutic intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcoholic liver disease is the leading cause of liver-related morbidity and mortality worldwide and is a major cause of death among adults with prolonged alcohol abuse [1]. According to the World Health Organization, 3.3 million deaths occur worldwide every year due to the harmful use of alcohol, representing 5.9 % of all deaths. Alcohol is the third leading preventable cause of death in the US accounting for about 88,000 deaths per year [2, 3]. This review highlights the natural history, pathogenesis, molecular and cellular targets, as well as the current and new therapies being investigated for Alcoholic Hepatitis.
Alcoholic liver disease encompasses different stages of liver disease as a consequence of susceptibility factors and duration of alcohol consumption; listed from least to most severe, are steatosis, alcoholic steatohepatitis, progressive fibrosis, end stage cirrhosis, decompensated cirrhosis, and superimposed hepatocellular carcinoma (HCC).
Alcoholic hepatitis (AH) is an acute-on-chronic condition, diagnosed clinically by new-onset jaundice and/or ascites in the setting of ongoing alcohol abuse and underlying alcoholic liver disease (ALD). Severe forms of AH have very high short-term mortality and represent one of the deadliest diseases in clinical hepatology, with a mortality rate of 30–50 % at 3 months [4].
The true incidence of AH is not well known; population-based studies estimate approximately 4.5 hospitalizations for AH per 100,000 persons per year [5]. Patients with ALD can present with acute episodes of jaundice and liver decompensation from other reasons, such as sepsis, biliary obstruction, diffuse HCC, drug-induced liver injury and ischemic hepatitis. All the etiologies stated above present with a similar clinical picture and there is a lack of biomarkers or other laboratory tests to distinguish these acute entities. Where diagnosis is unclear, transjugular liver biopsies in patients hospitalized for acute hepatitis with underlying ALD to confirm the existence of AH is important.
Environmental and genetic risk factors
There is a positive correlation between cumulative alcohol intake and degree of liver fibrosis; however, extensive variability in the histological response to alcohol abuse exists in individuals. At similar levels of ethanol consumption, some patients only develop fatty liver or macrovesicular steatosis, while others progress to fibrosis and cirrhosis. Several risk factors for the susceptibility of ALD have been identified including sex, obesity, drinking patterns, dietary factors, non-sex-linked genetic factors, and cigarette smoking [1, 6, 7] (Fig. 1).
Natural progression along the spectrum of ALD, from steatosis, to the inflammatory state of steatohepatitis, to progressive fibrosis and cirrhosis, and, finally, to decompensated cirrhosis and HCC. Exacerbations of AH occur at many of the later stages of disease. Predisposing risk factors to accelerated progression are listed
Epidemiological studies suggest that several genetic factors influence the severity of steatosis and oxidative stress, and that the cytokine milieu, the magnitude of the immune response, and the severity of fibrosis also modulate an individual’s propensity to progress to advanced ALD. The genetic factors that influence an individual’s susceptibility to develop advanced ALD are largely unknown. Variations in genes encoding antioxidant enzymes, cytokines, other inflammatory mediators, and alcohol-metabolizing enzymes seem to play a role [7].
Owing to its fibrogenic potential, variations in the rate of generation of acetaldehyde could explain the differences in the susceptibility of individuals to ALD after abusive alcohol consumption. Although polymorphisms in the genes encoding the main alcohol-metabolizing enzymes such as ADH, acetaldehyde dehydrogenase and cytochrome P450 2E1 (CYP2E1) are accepted to be involved in an individual’s susceptibility to alcoholism, their role in the progression of ALD remains controversial [8].
Also, recent studies indicate that variations in the patatin-like phospholipase domain-containing protein 3 (PNPLA3) strongly influence the development of cirrhosis in alcoholic Caucasians and Mexicans. PNPLA3 polymorphisms can be considered as the only confirmed and replicated genetic risk factor for ALD. However, deletion of the PNPLA3 gene did not affect obesity-associated fatty liver or liver enzyme elevation in animal studies [9–11].
Polymorphisms in genes that encode pro-inflammatory cytokines like tumor necrosis factor (TNF), interleukin-1 receptor-associated kinase, interleukin-1β, IL-1 receptor antagonists, IL-2, IL-6 and IL-10, involved in the pathogenesis of ALD, have also been examined [12]. Moreover, studies have also investigated the role of genetic variation in factors involved in lipopolysaccharide-induced intracellular pathways, including CD14 and toll-like receptor 4 (TLR4), as potential risk factors for ALD [13].
Despite the large number of studies that have assessed the role of genetic variation in susceptibility to ALD, a large-scale, well-designed, genome-wide association study of factors linked to the development of ALD remains to be performed. Consequently, a genetic test capable of identifying which patients are susceptible to advanced ALD is yet to be developed. Such a test could be useful in clinical settings, as it would help to identify the main genetic determinants of ALD, which could potentially assist in the development of future therapies [14].
Current treatment in management of alcoholic hepatitis
Patients that develop severe AH usually require hospitalization for initial management; a summary of current interventions is listed in Table 1. Primary prevention is aimed at alcohol abstinence and an active management of alcohol use disorders is critical to achieving continued abstinence. For the successful management of these patients, a multidisciplinary team composed of hepatologists, psychologists, psychiatrists and social workers is highly recommended [15]. Significant protein calorie malnutrition is a common finding in alcoholics, as are deficiencies in a number of vitamins and trace minerals, including vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc [1, 16, 17]. Nutritional support improves liver function, and short-term follow-up studies suggest that improved nutrition might improve survival times and histological findings in patients with AH [18]. Most patients improve spontaneously with abstinence and supportive care. Medical treatment is considered for patients who present with a very severe clinical picture or continue to deteriorate (Fig. 2).
Clinical evaluation for a patient with high suspicion of AH involves ruling out precipitating factors of decompensated liver disease and confounding illnesses. The role of transjugular liver biopsy is dependent on the care provider’s confidence in the diagnosis. Initial treatment requires ongoing surveillance for improvement
Several clinical scoring models have been developed to help predict outcomes of patients with AH and to guide therapy, including the Maddrey discriminant function (DF); Glasgow Alcoholic Hepatitis Score (GAHS); Mayo End-stage Liver Disease (MELD); Age, Bilirubin, INR, Creatinine (ABIC); MELD-Na, UK End-stage Liver Disease, and three scores of corticosteroid response at 1 week: an Early Change in Bilirubin Levels, a 25 % fall in bilirubin, and the Lille score. The MELD, DF, GAHS, ABIC and scores of corticosteroid response prove to be valid in an independent cohort of biopsy-proven alcoholic hepatitis [19]. Combining features of various scoring models, for example, from the DF, ABIC, MELD and Lille, may prove to be a better prognosis indicator [20].
Corticosteroids have been used in the treatment of AH for more than 40 years. Prednisolone is widely considered the first line therapy for severe AH. Both the AASLD and EASL practice guidelines recommend the use of corticosteroids (i.e. prednisolone 40 mg daily for 4 weeks) for patients with severe AH, defined by Maddrey’s discriminant function >32 or the presence of hepatic encephalopathy [1, 21]. Clinical trials conducted, to determine efficacy of steroid administration, so far suffer from heterogeneity and a high risk of bias. A recent meta-analysis from individual data, however, showed improved survival in patients with a high DF. In this study, patients were categorized as complete responders, partial responders, or null responders, and it was possible to predict the 6-month survival of each group using 2 new cut-offs of the Lille score [22]. In another study, the response to prednisolone was assessed based on the change in bilirubin after 1 week of therapy and quantified using the Lille score. The Lille score was calculated after 7 days of initiation of therapy, and it was determined that corticosteroids can be discontinued in non-responders, defined by a Lille score >0.4520. A Lille score >0.45 predicts a 6-month survival rate of <25 % [23]. Clinical practice guidelines recommend stopping corticosteroids after 1 week in those with an unfavorable Lille score, as the risks of continued therapy likely outweigh the benefits. The contraindications for the use of corticosteroids are not well defined. When considering treatment with corticosteroids, patients require careful monitoring for evidence of present or developing infections and/or active GI bleeding.
Pentoxifylline is a phosphodiesterase inhibitor that inhibits the synthesis of tumor necrosis factor, which is increased in patients with AH. In practice, pentoxifylline is typically reserved as a second-line agent for patients with contraindications to corticosteroid therapy. A large trial, STOPAH, comparing prednisolone and pentoxifylline, is underway and should prove to be a definitive study for assessing the efficacy of these drugs for AH [24]. Current consensus regarding Pentoxifylline is that it is not an effective rescue therapy in patients who do not respond to corticosteroids [25].
Infliximab and etanercept, anti-TNF agents, were also investigated as potential therapies for patients with AH. Rationale for their use was similar to pentoxifylline; TNF-α was implicated as a key culprit in the potentiation of hepatocyte inflammation. The use of these agents should theoretically benefit patients hospitalized with AH. However, translational studies did not support the hypothesis [26, 27]. Other larger studies resulted in adverse side effects such as increased rates of infection and increased mortality. Presently, anti-TNF-α agents are not recommended for treatment of AH [28].
N-acetylcysteine is known to replenish glutathione in damaged hepatocytes and prevent cell death in ALD. A recent randomized trial showed that the combination of N-acetylcysteine with prednisolone showed a clear trend to improve survival, by reducing 1-month mortality (8 vs. 24 %) and reduce incidence of hepatorenal syndrome and infection, although the study was underpowered to reach statistical significance. In addition, it was found to have no effect on 6-month survival [29]. The favorable safety profile of N-acetylcysteine makes it a potential option, in combination with corticosteroids, for patients with severe disease.
Role of transplantation in alcoholic hepatitis
ALD is the second most common indication for liver transplantation (LT) for chronic liver disease after hepatitis C virus cirrhosis. Despite this, it is estimated that as many as 95 % of patients with end-stage liver disease related to alcohol are never formally evaluated for candidacy for liver transplantation.
An important issue that is still unresolved is the role of LT in patients with alcoholic hepatitis, who are generally excluded from transplant. Patients with severe AH who do not respond to medical therapy are unlikely to survive the ‘‘mandatory” 6-month abstinence period as their risk of mortality is quite high [30, 31]. However, post-transplant outcomes appear to be good for highly selected patients with severe AH unresponsive to medical therapy with low rates of alcohol relapse [32, 33]. Salvage liver transplantation in highly selected patients has been shown to significantly improve survival, but is not available in the vast majority of transplant centers [32]. The availability of living donor transplantation and extended criteria donor liver transplantation are likely to heighten the debate on this issue.
Translation research in ALD: integrating animal and human studies
While animal models serve as the cornerstone of many research studies, their use in exploring potential novel targets for the treatment of alcoholic liver disease, particularly alcoholic hepatitis, is limited. Many rodent models produce some features of chronic alcoholic liver disease, but it is difficult to mimic acute-on-chronic liver injury as seen in patients with alcoholic hepatitis, though improved models are emerging.
While many models exist to mimic mild to moderate levels of hepatocyte damage, a better model of alcoholic hepatitis is the chronic-plus-binge feeding model. As the name implies, it models the chronic drinking behaviors with intermittent binges as seen in patients presenting with alcoholic hepatitis. This model causes hepatocyte damage, disruption of mitochondrial function, and oxidative stress, resulting in moderate rises in AST and ALT, but, in addition to mimicking steatosis, liver injury, and early fibrogenic response, it was also able to demonstrate hepatic neutrophil infiltration as seen in early stages of alcoholic hepatitis [34], though the hepatocellular damage and inflammation were noted to be transient. Given the prevalence of obesity, rodent models have been developed to encompass both non-alcoholic steatohepatitis and alcoholic liver disease. The hybrid model with high-fat and high-cholesterol plus chronic and binge ethanol feeding involves a high-fat and high-cholesterol diet comprising 40 % of the caloric intake, while chronic intragastric ethanol feeding comprised 60 % of the caloric intake of these mice, and was supplemented by weekly binges of ethanol. This model represents moderate to severe alcoholic hepatitis, causing significant liver inflammation, by reproducing chronic alcoholic steatohepatitis characterized by balloon cell degeneration, macrophage activation and infiltration, and progression of liver fibrosis. It closely mimics alcoholic hepatitis histologically, with findings of neutrophil infiltration, but also clinically represents this acute entity as mice develop splenomegaly, hypoalbuminemia, and hyperbilirubinemia [35]. Models to show advanced fibrosis and cholestasis, as observed in alcoholic hepatitis, do not yet exist. “Second-hit” or “multiple-hit” models exist which use chronic ethanol feeding to induce hepatic susceptibility to other agents, including nutritional modification or pharmacologic agents to achieve the acute-on-chronic disease state, have shown some promising pathologic similarities to human alcoholic hepatitis including coagulative necrosis and inflammation [36], but often as a consequence of the second agent used, and this calls into question their clinical relevance.
The traditional approach of identifying molecular drivers in animal models and translating this work to human disease poses many challenges. In addition, the challenges of finding appropriate models to mimic histologic findings in human alcoholic liver disease and incorporate co-morbidities faced by patients with alcoholic liver disease, and those presenting with alcoholic hepatitis, remain difficult to do.
A systematic rational approach to designing translational studies to examine alcoholic hepatitis begins by first determining the phenotype of the patient, disease severity and comorbidities that contribute to the acute disease state, with careful consideration given to related overlapping syndromes. Disease severity and patient prognosis can be computed using a variety of scoring systems as discussed previously. The second step involves obtaining anthropometrical and clinical data, histological data from liver biopsy specimens, and data from genomic, proteomic, and metabolomic studies performed on ancillary biospecimens including suprahepatic and peripheral blood, polymorphonuclear leukocytes, and stool. The third step involves the correlation of gene and protein expression in biospecimens obtained with clinical or histological features observed in patients to identify potential molecular drivers of disease. Finally, the fourth step involves testing hypothetical relations in in vitro and in vivo models in carefully designed animal studies, with the eventual goal in mind of identifying druggable molecular targets to reverse or abort progression of this disease (Fig. 3).
Molecular pathogenesis of alcoholic liver disease and potential targets for intervention
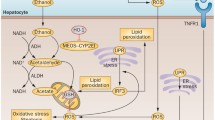
An understanding of the spectrum of disease states comprising ALD and the pathogenic mechanisms at work is imperative to our understanding of the development of alcoholic hepatitis as an acute-on-chronic state, and provides ample opportunity to halt progression in early disease stages. Table 2 summarizes some of the key molecular and cellular markers that are potential new targets for therapeutic intervention. Chronic alcohol ingestion leads to steatosis, an asymptomatic state reversible on cessation of alcohol consumption. Prolonged alcohol consumption can lead to alcoholic steatohepatitis, and an inflammatory state marked by neutrophil infiltration and hepatocellular damage. Histological evaluation demonstrates fat accumulation, hepatocyte ballooning, neutrophil infiltration, and even early signs of pericellular and sinusoidal fibrosis. Fibrosis can then progress to cirrhosis complicated by poor synthetic function portal hypertension and its associated complications. Alcoholic hepatitis, as previously mentioned, is an acute-on-chronic disease state, in which the majority of patients have underlying advanced fibrosis, and, in addition to a marked inflammatory response, histologically, canalicular and lobular bilirubinostasis may be present [37]. The interplay of molecular and cellular markers contributing to disease is complex (Fig. 4) and is discussed in detail below.
Steatosis as a result of decreased fatty acid oxidation
Steatosis evolves from the intrahepatic accumulation of fats, mainly triglycerides, phospholipids and cholesterol esters, which is aggravated by excessive alcohol intake through disruption of fatty acid oxidation, and increase of fatty acid and triglyceride synthesis and uptake. Alcohol intake increases the NADH/NAD+ ratio in hepatocytes, which disrupts mitochondrial β-oxidation of fatty acids resulting in accumulation and steatosis [38]. Excessive alcohol consumption is also responsible for decreasing β-oxidation of fatty acids through its metabolite, acetaldehyde, which directly inhibits the DNA-binding ability and transcriptional activation ability of peroxisome proliferator-activated receptor-α (PPAR-α) [ 38], a nuclear hormone receptor that regulates transcription of many genes involved in free fatty acid transport and oxidation [39, 40]. Ethanol can also indirectly inhibit the function of PPAR-α through various mechanisms. The first is by upregulating cytochrome P450 2E1 (CYP2E1) and hepatic oxidative stress, which in turn inhibits oxidation of fatty acids by preventing upregulation of PPAR-α as demonstrated in wild-type mice as compared to CYP2E1 knock-out mice [41]. The second and third include downregulation of adiponectin and zinc, both of which have been demonstrated to downregulate PPAR-α [42, 43].
Steatosis as a result of increased fatty acid uptake and synthesis
It is well known that alcohol consumption increases hepatic influx of free fatty acids from adipose tissue and chylomicrons from the intestinal mucosa [38]. Fatty acid synthesis is increased through upregulation of lipogenic enzymes. This is mainly accomplished directly through upregulation of sterol regulatory element-binding protein-1c (SREBP-1c), a master transcription factor that upregulates the expression of genes encoding lipogenic enzymes, or indirectly through inhibition of factors that inhibit SREBP-1c. Acetaldehyde promotes transcription of SREBP-1c, which in turn upregulates expression of lipogenic enzyme genes, contributing to increased fatty acid synthesis [44]. SREBP-1c is also upregulated through multiple other processes including ethanol-induced hepatocyte ER stress [45], production and binding of adenosine to A1 receptors [46], and through endocannnabinoids [47]. Endocannabimoids mediate alcoholic liver injury through signaling either of two cannabinoid receptors, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) [48]. Mouse studies have demonstrated that signaling through the CB1 cannabinoid receptor worsens alcoholic liver injury, while signaling through the CB2 confers a protective role in the development of steatosis and alcoholic liver injury [47]. This suggests that CB1 receptor antagonsists and CB2 receptor agonists could serve as potential therapeutic agents in preventing steatosis and alcoholic liver disease. CB1 receptor antagonists have their limitations, due to their neuropsychiatric side effects; however, peripherally acting CB1 antagonists are currently being explored [48]. SREBP-1c is upregulated indirectly through downregulation of various factors that reduce its expression, such as ethanol-mediated AMP-activated protein kinase (AMPK) inhibition [49]. Studies in rat hepatoma cells demonstrated that activation of SREBP-1c by ethanol is also mediated by mammalian sirtuin 1 (SIRT1), a NAD(+)-dependent class III protein deacetylase; ethanol exposure induced SREBP-1c lysine acetylation and SREBP-1c transcriptional activity, which was reversed by adding a SIRT1, suggesting that ethanol must have an inhibitory effect on SIRT1, inhibiting its inhibitory effect on SREBP-1c [50]. This finding suggests that SIRT1 agonists could potentially be used to curtail the effects of ethanol on steatosis. To extend this further, it has been shown that adiponectin confers protection against alcoholic fatty liver via modulation of complex hepatic signaling pathways through the central regulatory system, the SIRT1–AMPK axis [51].
Direct effects of ethanol on enzymes involved in fatty acid metabolism
In addition to regulating transcription factors involved in the regulation of genes involved in fatty acid metabolism, ethanol can also directly affect the activities of these enzymes through its inhibition of AMPK, a serine-threonine kinase. This was demonstrated through animal models of concomitant obesity and alcohol consumption, in which these mice demonstrated defective adiponectin-AMPK signaling even in the presence of increased adiponectin downstream of p-AMPK, suggesting a role for AMPK in steatosis [52]. AMPK functions to inactivate the rate-limiting enzyme acetyl-CoA carboxylase (ACC) in fatty acid synthesis. In turn, inactivation of ACC leads to a reduction in the levels of malonyl Co-A, a precursor in fatty acid synthesis, and an inhibitor of the rate-limiting enzyme carnitine palmitoyltransferase 1 (CPT1), in fatty acid oxidation, resulting in less fatty acid synthesis and increased oxidation [53].
In addition, AMPK directly phosphorylates and inhibits SREBP activity in hepatocytes, thereby attenuating steatosis. In this manner, AMPK inhibits fatty acid synthesis but promotes fatty acid oxidation via the inactivation of ACC enzyme activity [54]. Alcohol consumption inhibits AMPK activity in the liver, leading to increased activity of ACC and decreased activity of CPT1, contributing to hepatic fatty acid accumulation and progression to steatosis [49].
Cellular pathogenesis of alcoholic liver disease
The role of autophagy in steatosis
Autophagy removes lipid droplets from hepatocytes [55]. The role of alcohol intake on the autophagic process depends greatly on the chronicity or acuity of intake. Chronic alcohol consumption inhibits autophagy, and thus leads to fat accumulation and steatosis [56, 57]. Mouse studies have demonstrated that acute ethanol consumption conversely activates autophagy through the production of reactive oxygen species (ROS) and inhibition of the mammalian target of rapamycin (mTOR) signaling pathway, inhibiting progression to steatosis [58].
The role of the innate immune system in development of steatohepatitis
The innate immune system plays a large role in the propagation of inflammation in steatohepatitis and the development of fibrosis. Excessive alcohol intake results in the mass production of ROS, which react with nucleic acids, fatty acids, proteins, and structural cell components to form adducts. These adducts are known to be potent activators of the innate immune system [59].
The contribution of bacterial-immune system interplay has received more attention in many gastrointestinal disorders. Kupffer cells, the liver’s native phagocytes, serve as the first line of innate immune system defense. It is known that alcohol increases gut permeability resulting in translocation of bacterial products such as LPS into portal circulation. This results in activation of Kupffer cells through TLR4 signaling through MyD88-dependent and -independent (TRIF/IRF-3) pathways [60], leading to the production and release of pro-inflammatory cytokines, including TNF-α [61–63]. TLR antagonists have been proposed as potential therapeutic agents for the management of alcoholic liver disease. Kupffer cells are also activated through binding of complement proteins, C3 and C5, induced through ethanol activation of the complement cascade [64, 65].
While the innate inflammatory response is generally believed to propagate liver injury, Kupffer cells also have mechanisms in place to slow down and halt progression of inflammation through secretion of anti-inflammatory cytokines, including IL-6 and IL-10. Through activation of STAT3, these cytokines curb inflammation and slow progression of liver injury [66–68].
Neutrophil infiltration is cytokine-mediated, through IL-17; IL-17 also stimulates hepatic stellate cells (HSCs) to produce IL-8 and CXCL1, and these chemokines in turn recruit more neutrophils, completing the cycle. Additional mediators that assist with neutrophil recruitment include IL-1, osteopontin, CXCL4, CXCL5, and CXCL6; these also activate macrophages during liver injury [68, 69].
The role of the innate immune system in development of fibrosis
Hepatic fibrosis is characterized by the excessive accumulation of collagen and other extracellular matrix proteins. The extensive hepatocellular damage that occurs at this stage of liver injury results in the production of cytokines, neuroendocrine factors, and angiogenic factors, leading to activation of HSCs. Portal and bone marrow-derived fibroblasts also contribute to fibrosis [70, 71].
The ethanol metabolite, acetaldehyde, plays an important role in the instigation and maintenance of fibrosis, through HSC activation and maintenance of the activated phenotype. Through its rapid reaction with cellular components to form adducts, acetaldehyde and its adducts, malondialdehyde, 4-hydroxynonenal, and malondialdehyde-acetaldehyde, act upon HSCs to keep them in the perpetually activated “on” state [72].
In a similar manner, ROS can cause activation of HSCs. ROS also directly stimulate fibrosis through activation of pro-fibrogenic signaling pathways in HSCs, including ERK1, ERK2, phosphinositide 3 kinase-Akt and JNK, which enhance collagen production [73]. ROS also propagate collagen accumulation by preventing collagen degradation, first, through direct inhibition of metalloproteinases which degrade collagen, and secondly, through upregulation of the tissue inhibitor of metalloproteinases [73].
Extrinsic propagators of fibrosis include transluminal translocation of bacterial LPS. LPS activates the TLR4 on HSCs directly inducing HSC activation [74], and activates TLR4 signaling pathways on hepatic sinusoidal endothelial cells promoting angiogenesis and subsequent fibrosis [75]. Finally, TLR4 indirectly stimulates fibrosis through activation of Kupffer cells which, in exchange, release ROS and other pro-fibrogenic cytokines, causing activation of HSCs [76, 77].
Cellular targets for therapeutic management of alcoholic hepatitis
Cell death via apoptosis
Massive hepatocyte cell death is a prominent feature of alcoholic hepatitis, and, as previously discussed, apoptosis is a prominent feature of many of the preceding stages of alcoholic liver disease. Since caspase inhibitors are known to inhibit apoptosis, animal studies have been carried out in models of chronic liver injury from viral hepatitis secondary to hepatitis C infection, and non-alcoholic steatohepatitis, and caspase inhibitors have shown promising results in ameliorating liver injury and impeding progression to fibrosis [78–80]. It is reasonable to think such an approach would work in alcoholic liver disease, in particular in alcoholic hepatitis.
Role of innate immune system
Studies from other models of liver disease suggest that, following activation, neutrophils undergo transmigration into the liver parenchyma where they destroy damaged hepatocytes through the release of ROS and proteases, supporting their prominent role in ALD [81]. IL-17 is increased in patients with alcoholic hepatitis and directly induces neutrophil recruitment, but also indirectly promotes neutrophil recruitment by stimulating HSCs to secrete IL-8 and CXCL [82, 83]. This suggests that the modification of these chemokines, or their precursors or activators, may mediate neutrophil infiltration and perhaps attenuate alcoholic hepatitis. Translational studies have examined the role of the CXCL family of chemokines, and found elevated levels correlate with severity of disease, degree of portal hypertension, and patient survival [84, 85]. Given these promising findings, therapeutic agents that target CXCL chemokines may be considered in the treatment of AH. Osteopontin is an extracellular matrix protein that is markedly upregulated in alcoholic hepatitis, similar to other CXCL chemokines [86]. Therefore, agents that inhibit osteopontin are also attractive in considering new therapeutic agents. The redundancy of chemokines and their receptors makes the development of targeted therapeutics challenging.
Instigators of inflammation are also thought to play an important role. Sources of inflammatory mediators can be classified as sterile, originating from intracellular sources, or microbiological, from bacterial translocation in the gut. Damage-associated molecular patterns (DAMPs) are intracellular molecules released by dying cells that trigger the innate immune system [87]. Among the DAMPs, high-mobility group box-1 (HMGB-1) has been implicated in the development of alcoholic steatohepatitis [88], and likely also has a role in alcoholic hepatitis. Gut-derived bacterial products belong to the class of pathogen-associated molecular patterns (PAMPs). These PAMPs circulate through the portal circulation and induce an inflammatory response through activation of HSCs and Kupffer cells [88, 89]. Inhibition of gut leakage could be a potential target for therapy aimed at preventing the initiation of the innate immune response in alcoholic hepatitis.
Role of the adaptive immune system
While the role of the innate immune system has been widely explored, the role of the adaptive immune system in hepatocellular injury and propagation of alcoholic hepatitis leaves many questions unanswered. It is well known that the adaptive immune system responds to oxidative stress and peroxidation adducts, but its role in hepatocellular damage and inflammation in alcoholic hepatitis remains unknown. As previously described, increased alcohol consumption generates ROS through multiple mechanisms and leads to adduct formation; protein adducts have altered conformation and function, and are relatively immunogenic. Patients with alcoholic hepatitis have been found to have circulating T cells with antibodies to these adducts, enforcing that the adaptive immune response likely plays a large but as yet undiscovered role in AH [90–93].
Targeting dysbiosis
Alterations in the gut microbiome have unique implications on the development of alcoholic hepatitis. This was first suggested in the intragastric mouse feeding model in which elevated serum ethanol levels were maintained; treated mouse populations were observed to have both microbial translocation and dysbiosis [94]. In studies involving patients with chronic alcoholic liver disease, administration of probiotics appeared to improve liver function in this patient group, further supporting that the intestinal bacterial milieu is of great importance [95]. Work examining the applicability of probiotics in patients with alcoholic hepatitis is still underway. Other studies which included genomic and metabolomic analyses of intestinal bacteria revealed low levels of lactobacilli and reduced production of saturated long-chain fatty acids (LCFA). In this model, supplementation with LCFA restored eubiosis, intestinal barrier function, and reduced liver injury in mice [96], suggesting a role for potential supplementation of LCFA in this patient group.
The role of hepatocyte proliferation and regeneration
Hepatic regeneration in the healthy liver results from expansion of the remaining healthy hepatocytes [97]. In the diseased state, in which hepatocyte proliferation is inhibited [98], pluripotent liver progenitor cells, also referred to as oval cells or ductal hepatocytes, proliferate and differentiate to repopulate hepatocytes or biliary epithelial cells [99]. In the rodent model, alcohol attenuates regeneration of hepatocytes following partial surgical hepatectomy [100], so, although human studies are lacking, it is reasonable to hypothesize that alcohol not only causes hepatocellular injury and death but also prevents regeneration. While histologically the presence of bilirubinostasis and severe fibrosis are associated with a poorer prognosis in alcoholic hepatitis, the presence of proliferating hepatocytes is associated with better prognosis [101]. In addition, intense neutrophilic infiltrate was also associated with better prognosis [99], suggesting that cytokines released by neutrophils likely play a role in hepatic regeneration following cessation of alcohol, and that resolving inflammation may actually have a beneficial, rather than detrimental role in alcoholic liver disease, contributing to hepatic regeneration. Severe alcoholic hepatitis is marked by failure of liver progenitor cells to progress past massive proliferation to maturation into mature hepatocytes [102]; the mechanism for this remains to be elucidated. Potential therapeutic agents to promote hepatic regeneration are being explored.
Conclusion
ALD is a leading cause of liver-related morbidity and mortality, encompassing a spectrum of disorders including asymptomatic steatosis, steatohepatitis, fibrosis, cirrhosis and its related complications, as well as the acute-on-chronic state of AH. While multidisciplinary efforts continue to be aimed at curbing progression of this spectrum of disorders, there is an urgent need for effective therapeutic interventions for AH given its high mortality rate and the limitations of current treatment regimens. Corticosteroids and pentoxyfylline, though used extensively, offer only a modest survival benefit. Anti-TNF agents, including infliximab and etanercept, have not proved to be safe or effective, due to the increased rates of infection and increased mortality observed in larger studies. Adjunctive therapies, such as N-acetylcysteine, which is known to replenish glutathione in damaged hepatocytes and prevent cell death in ALD, was also found to have no effect on 6-month survival. Liver transplantation continues to be limited for patients with ALD, and though the risk of associated complications is not higher among this group, there remains a hesitancy and concern among providers, and it is estimated that as many as 95 % of patients with end-stage liver disease related to alcohol are never formally evaluated for candidacy for LT. This drives our need to find better therapeutic targets for ALD with the focus on AH. While our understanding of the molecular and cellular mechanisms of disease stems from animal models, there remains a dire need for translational research in this field to aid in bridging our gaps in understanding. Recent work in discovering the molecular and cellular basis of disease progression in ALD has helped uncover potential new targets for therapeutic intervention. In early stages of ALD, particularly in steatosis, many molecular targets involved in modifying transcription of genes responsible in fatty acid synthesis and accumulation have been explored as potential targets of therapeutic intervention, including SREBP-1c and the cannabinoid receptors, CB1 and CB2. Similar pathways, including activation of the receptor PPAR-α, have been explored in their role in fatty acid oxidation as it relates to progression to steatosis. The roles of various inflammatory pathways in progression to steatohepatitis have been explored as potential targets, including inhibition of translocation of bacterial products, such as LPS, which are known to trigger an inflammatory response, inhibition of TLR signaling pathways, and release of pro-inflammatory cytokines, including IL-6 and IL-10. Inhibition of profibrogenic pathways in HSCs, including ERK1, ERK2, phosphinositide 3 kinase-Akt and JNK, have also been explored and are attractive candidates for therapeutic intervention to prevent progression to fibrosis. Much work remains to elucidate the role of the cellular responses of the innate and adaptive immune systems in AH in uncovering potential targets for intervention. The role of IL-17 in inducing neutrophil recruitment by stimulating HSCs to secrete IL-8 and CXCL1 has been explored, with the thought that modification of these chemokines may mediate neutrophil infiltration and attenuate alcoholic hepatitis. The role of osteopontin has also been explored as it contributes to neutrophillic recruitment in alcoholic hepatitis, but much work remains ahead. Similarly, the mechanisms have not yet been elucidated, but it is well known that the adaptive immune response plays an important role in progression to AH, as antibodies to adducts generated through free radical oxidation have been found on circulating T cells, though potential targets for therapeutic intervention remain to be discovered.
Abbreviations
- AASLD:
-
American Association for the Study of Liver Disease
- ASH:
-
Alcoholic steatohepatitis
- ABIC:
-
Age, Bilirubin, INR, Creatinine
- ADH:
-
Alcohol dehydrogenase
- AH:
-
Alcoholic hepatitis
- ALD:
-
Alcoholic liver disease
- AMPK:
-
AMP-activated protein kinase
- CYP2E1:
-
Cytochrome P450 2E1
- DF:
-
Maddrey discriminant function
- EASL:
-
European Association for the Study of the Liver
- ER:
-
Endoplasmic reticulum
- GAHS:
-
Glasgow Alcoholic Hepatitis Score
- HCC:
-
Hepatocellular carcinoma
- HSC:
-
Hepatic stellate cell
- IFN:
-
Interferon
- IRAK-M:
-
Interleukin-1 receptor-associated kinase
- IL:
-
Interleukin
- MELD:
-
Model for end-stage liver disease
- LPS:
-
Lipopolysaccharide
- LT:
-
Liver transplant
- NIAA:
-
National Institute of Alcohol Abuse and Alcoholism
- NK:
-
Natural killer
- PMN:
-
Polymorphonuclear leukocytes
- PNPLA3:
-
Patatin-like phospholipase domain-containing protein 3
- PPAR:
-
Peroxisome proliferator-activated receptor
- ROS:
-
Reactive oxygen species
- SAMe:
-
S-adenosylmethionine
- SREBP-1c:
-
Sterol regulatory element-binding protein 1c
- TLR:
-
Toll-like receptor
- TLR4:
-
Toll-like receptor 4
- TNF:
-
Tumor necrosis factor
- UKELD:
-
UK End stage liver disease
- WHO:
-
World Health Organization
References
O’Shea RS, et al. Alcoholic liver disease. Hepatology 2010;51:307–328
World Health Organization. Alcohol Fact Sheet. WHO Media Center Fact Sheets 2015 http://www.who.int/mediacentre/factsheets/fs349/en/
NIAAA. Alcohol Facts and Statistics. http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics
Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol 2015;62(1 Suppl):S38–S46
Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999–2008: a nationwide population based cohort study. J Hepatol 2011;54:760–764
Tsukamoto H, et al. “Second hit’’ models of alcoholic liver disease. Semin Liver Dis 2009;29:178–187
de Wilfred Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis 2007;27:44–54
Zintzaras E, et al. Do alcohol-metabolizing enzyme gene polymorphisms increase the risk of alcoholism and alcoholic liver disease? Hepatology. 2006;43:352–361
Tian C, et al. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet 2010;42:21–23
Stickel F, Hampe J. Genetic determinants of alcoholic liver disease. Gut. 2012;61:150–159
Trépo E, et al. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. J Hepatol 2011;55:906–912
Marcos M, et al. Tumor necrosis factor polymorphisms and alcoholic liver disease: a HuGE review and meta-analysis. Am J Epidemiol 2009;170:948–956
Zeng T, et al. Association between CD14-159C>T polymorphisms and the risk for alcoholic liver disease: a meta-analysis. Eur J Gastroenterol Hepatol 2013;25:1183–1189
Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol 2011;8:491–501
Casanova J, Bataller R. Alcoholic hepatitis: prognosis and treatment. Gastroenterol Hepatol 2014;37:262–268 Epub 2014 Mar 20
Singal AK, et al. Nutritional status of patients with alcoholic cirrhosis undergoing liver transplantation: time trends and impact on survival. Transpl Int 2013;26:788–794
Rambaldi A, et al. Anabolic-androgenic steroids for alcoholic liver disease: a Cochrane review. Am J Gastroenterol 2002;97:1674–1681
Cabré E, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32:36–42
Papastergiou V, et al. Nine scoring models for short-term mortality in alcoholic hepatitis: cross-validation in a biopsy-proven cohort. Aliment Pharmacol Ther 2014;39:721–732
Louvet A, et al. Combining data from liver disease scoring systems better predicts outcomes of patients with alcoholic hepatitis. Gastroenterology. 2015;149:398–406.e8 Epub 2015 Apr 29
European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012;57:399–420
Mathurin P, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–260
Louvet A, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354
Forrest E, et al. Steroids or pentoxifylline for alcoholic hepatitis (STOPAH): study protocol for a randomised controlled trial. Trials. 2013;14:262
Whitfield K, et al. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009;4:1–46
Colmenero J, Bataller R, Sancho-Bru P, et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687–697
Dominguez M, Miquel R, Colmenero J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–1650
Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–1960
Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011;365:1781–1789
Stickel F, Seitz HK. Alcoholic steatohepatitis. Best Pract Res Clin Gastroenterol 2010;24:683–693
Dureja P, Lucey MR. The place of liver transplantation in the treatment of severe alcoholic hepatitis. J Hepatol 2010;52:759–764
Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790–1800
Burra P, Senzolo M, Adam R, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant 2010;10:138–148
Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 2013;8:627–637
Lazaro R, Wu R, Lee S, Zhu NL, Chen CL, French SW, et al. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology. 2015;61:129–140
Tsukamoto H, Machida K, Dynnyk A, Mkrtchyan H. “Second hit” models of alcoholic liver disease. Semin Liver Dis 2009;29:178–187
Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol 2011;8:491–501
Baraona E, Lieber CS. Effects of ethanol on lipid metabolism. J Lipid Res 1979;20:289–315
Yu S, Rao S, Reddy JK. Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr Mol Med 2003;3:561–572
Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53:1023–1034
Lu Y, Zhuge J, Wang X, et al. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494
You M, Considine RV, Leone TC, et al. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577
Kang X, Zhong W, Liu J, et al. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50:1241–1250
You M, Fischer M, Deeg MA, et al. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem 2002;277:29342–29347
Esfandiari F, Medici V, Wong DH, et al. Epigenetic regulation of hepatic endoplasmic reticulum stress pathways in the ethanol-fed cystathionine beta synthase-deficient mouse. Hepatology. 2010;51:932–941
Peng Z, Borea PA, Varani K, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Invest 2009;119:582–594
Jeong WI, Osei-Hyiaman D, Park O, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab 2008;7:227–235
Tam J, Liu J, Mukhopadhyay B, et al. Endocannabinoids in liver disease. Hepatology. 2011;53:346–355
You M, Matsumoto M, Pacold CM, et al. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808
You M, Liang X, Ajmo JM, et al. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 2008;294:G892–G898
You MRC. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 2009;2009:850–859
Xu J, Lai KK, Verlinsky A, Lugea A, French SW, Cooper MP, et al. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol 2011;55:673–682
Viollet BGB, Leclerc J, Hébrard S, Lantier L, Mounier R, Andreelli F, Foretz M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 2009;196:81–98
Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 2011;13:376–388
Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology. 2011;140:1895–1908
Donohue TM Jr. Autophagy and ethanol-induced liver injury. World J Gastroenterol 2009;15:1178–1185
Wu D, Wang X, Zhou R, et al. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem Biophys Res Commun 2010;402:116–122
Ding WX, Li M, Chen X, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–1752
Chedid A, Arain S, et al. The immunology of fibrogenesis in alcoholic liver disease. Arch Pathol Lab Med 2004;128:1230–1238
Zhao XJ, Dong Q, Bindas J, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol 2008;181:3049–3056
Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract 2010;2010
Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952
Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231
Pritchard MT, McMullen MR, Stavitsky AB, et al. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132:1117–1126
Roychowdhury S, McMullen MR, Pritchard MT, et al. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology. 2009;49:1326–1334
Horiguchi N, Wang L, Mukhopadhyay P, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–1158
Miller AMWH, Bertola A, et al. Inflammation-associated IL-6/STAT3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in IL-10 deficient mice. Hepatology. 2011;54:846–856
Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–197
Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest 2012;122:3476–3489
Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209–218
Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669
Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev 2010;3:178–185
Bataller R, Schwabe RF, Choi YH, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 2003;112:1383–1394
Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 2007;13:1324–1332
Jagavelu K, Routray C, Shergill U, et al. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52:590–601
Purohit V, Brenner DA. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology. 2006;43:872–878
Cubero FJ, Urtasun R, Nieto N. Alcohol and liver fibrosis. Semin Liver Dis 2009;29:211–221
Pockros PJ, Schiff ER, Shiffman ML, et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–329
Shiffman ML, Pockros P, McHutchison JG, et al. Clinical trial: the efficacy and safety of oral PF-03491390, a pancaspase inhibitor—a randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol Ther 2010;31:969–978
Ratziu V, Chojkiet M, Sheikh M, et al. Safety, tolerability and preliminary activity of GS-9450, a selective caspase inhibitor, in patients with non-alcoholic steatohepatitis. J Hepatol 2010;52:S38
Ramaiah SK, Jaeschke H. Hepatic neutrophil infiltration in the pathogenesis of alcohol-induced liver injury. Toxicol Mech Methods 2007;17:431–440
Maltby J, Wright S, Bird G, et al. Chemokine levels in human liver homogenates: associations between GRO alpha and histopathological evidence of alcoholic hepatitis. Hepatology. 1996;24:1156–1160
Lemmers A, Moreno C, Gustot T, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–657
Colmenero J, Bataller R, Sancho-Bru P, et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687–697
Dominguez M, Miquel R, Colmenero J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–1650
Seth D, Gorrell MD, Cordoba S, et al. Intrahepatic gene expression in human alcoholic hepatitis. J Hepatol 2006;45:306–320
Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172
Ge X, Antoine DJ, Lu Y, et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J Biol Chem 2014;289:22672–22691
Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis 2010;28:737–744
Albano EVM. Immuno mechanisms in alcoholic liver disease. Genes Nutr 2010;5:141–147
Mottaran E, Stewart SF, Rolla R, et al. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med 2002;32:38–45
Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis 2004;24:273–287
Thiele GM, Duryee MJ, Willis MS, et al. Autoimmune hepatitis induced by syngeneic liver cytosolic proteins biotransformed by alcohol metabolites. Alcohol Clin Exp Res 2010;34:2126–2136
Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105
ProKirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 2008;42:675–682
Chen P, Torralba M, Tan J, Embree M, Zengler K, Starkel P, et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015;148(203–214):e16
Michalopoulos GK. Liver regeneration. J Cell Physiol 2007;213:286–300
Saso K, Moehren G, Higashi K, et al. Differential inhibition of epidermal growth factor signaling pathways in rat hepatocytes by long-term ethanol treatment. Gastroenterology. 1997;112:2073–2088
Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146(1231–9):e1–e6
Dubuquoy L, Louvet A, Lassailly G, Truant S, Boleslawski E, Artru F, et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut 2015;64:1949–1960
Rubbia-Brandt L, Lin-Marq N, Clement S, Frossard JL, Goossens N, et al. Hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis. J Hepatol 2015;63:609–621
Sancho-Bru P, Altamirano J, Rodrigo-Torres D, et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 2012;55:1931–1941
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding sources
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (1U01AA021908-01) and P30 DK34987.
Rights and permissions
About this article
Cite this article
Arsene, D., Farooq, O. & Bataller, R. New therapeutic targets in alcoholic hepatitis. Hepatol Int 10, 538–552 (2016). https://doi.org/10.1007/s12072-015-9701-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-015-9701-6