Abstract
This review focuses on iron metabolism, the genetics of hemochromatosis, current treatment protocols and various screening methods. Even though the most common form of hereditary hemochromatosis, C282Y gene mutations in the HFE gene, has been extensively studied, novel mutations in both HFE and non-HFE genes have been implicated in this disease. These have important implications for the Asia-Pacific region. In overload, deposition of iron in various body tissues leads to toxic damage. Patients commonly present with non-specific symptoms of malaise and lethargy. Biochemical, imaging and genetic testing can be carried out to confirm diagnosis. Venesection forms the mainstay of treatment and at present cascade screening of affected families is recommended over population-level screening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hereditary hemochromatosis (HH) is an inherited disorder of iron metabolism. It is among the most common autosomal recessive conditions of Caucasian populations [1, 2]. Resulting from abnormal regulation of iron absorption, excess dietary intake leads to increased body iron stores. The subsequent sequestration of iron in organs leads to tissue damage and eventually symptomatic disease [3, 4].
The history of hemochromatosis started with the observation of “chlorosis” in 1554 [5]. Then in the late 1800s Trousseau and Von Reckinghausen described “bronze diabetes”, which is now known to be clinically severe manifestations of hemochromatosis [6, 7]. Following this, Sheldon elucidated the link between iron metabolism and hemochromatosis pathogenesis, paving the way for identification of the mutation in the HFE gene, which results in the C282Y substitution in the HFE protein, in 1996 [8]. The current understanding of hemochromatosis focuses on the hepatic regulatory protein hepcidin and factors controlling its expression. At least four distinct subtypes of hemochromatosis have been recognised and described, each with distinct genetic and molecular profiles [9].
Diagnosis traditionally depended on coupling a clinically significant elevation in serum ferritin (SF) with C282Y homozygosity [10]. It is widely accepted that excess SF (>1,000 μg/l) can cause disease symptoms ranging from lethargy and fatigue, to endocrine dysfunction, to arthritis of the 2nd and 3rd metacarpophalangeal joints [4, 11]. However, the effect of mildly elevated SF between 300 and 100 μg/l is less clear; this is currently under investigation.
The mainstay of treatment for hemochromatosis, regular venesections, has remained unchanged over decades. Phlebotomy intervals are adjusted on an individual basis. Although there is some debate on the recommended maintenance range for SF, the current accepted standard remains at 50–100 μg/l [9, 12].
As HH is a hereditary condition with well-understood genetics, it initially appears to be the ideal candidate for population-level screening. However, only cascade screening of affected families is currently recommended.
Iron homeostasis
Iron’s crucial role in the body varies from primarily oxygen transport in hemoglobin and oxidative phosphorylation to being complexed in various other functional metalloproteins. However, it is also toxic in overload. With no regulated mechanism for excretion, uncontrolled loss (1–2 mg daily) in menses, bleeding and epithelial shedding, etc., are the only methods of iron removal. Due to this lack of control, excess iron uptake leads to the sequestration of iron in various tissues and organs. End-organ damage then results from increased redox-active availability of iron leading to oxidative damage to tissues through hydroxyl-free radicals via the Fenton and Haber-Weiss reactions [13–15].
Iron enters the body in both heme (animal protein) and non-heme (vegetable) form [16]. Both forms are absorbed by enterocytes in the duodenum and proximal small bowel. Non-heme iron can be either ferrous (Fe2+) or ferric (Fe3+). Ferric iron has to be reduced to ferrous prior to absorption, primarily because ferrous iron is more soluble [17], making it more readily absorbable [14]. Gastric acidity, duodenal cytochrome B (DCytB1) and other non-enzymatic pathways have been implicated in reducing ferric iron [14, 18]. Ferrous iron is taken up by the divalent metal transport 1 (DMT1) protein on the apical surface of enterocytes. This transporter is also used in the uptake of other divalent metals: manganese (Mn2+) and copper (Cu2+) [18]. Heme iron is taken up more efficiently by a hitherto unknown transport protein and brought into the enterocyte [19]. However, heme regulatory gene (HRG1) product and heme carrier protein (HCP1) have been implicated in heme iron intake.
Iron uptake in tissues is mediated by transferrin receptors (TfR1 and TfR2). Transferrin (Tf) binds to the TfR1 and is taken up into endosomes, where transferrin is cleaved and the receptor recycled back to the cell surface [20]. Excess iron in tissues is stored in complexes of hemosiderin or ferritin. Ferritin is a 24-subunit protein composed of heavy and light chains with the ability to carry approximately 4,500 compounded ferric ions [21]. Because ferritin is not directly implicated in the uptake process, a transient pool of redox-active free iron called the labile pool is known to exist. Hemosiderin, a by-product of ferritin degradation, is another iron storage molecule [14].
Ferroportin (FPN1) is the sole characterised iron exporter in cells. It is expressed on the basal surface and interacts with the ferroxidase hephestin to release iron into the circulation [22]. Once oxidised by hephestin, ferric iron is immediately bound by the transport molecule transferrin (Tf) [23]. Transferrin can exist as either holo- apo or di transferrin, depending on the iron saturation [14].
Ferroportin expression is negatively regulated by the small hepatic peptide, hepcidin [24]. Hepcidin binds to ferroportin, causing internalisation and degradation. This reduces the cells’ ability to export stored iron from enterocytes and other intracellular stores. The net result of hepcidin’s action is to reduce iron uptake and serum iron [14, 22].
The regulation of hepcidin release is complicated and has not been conclusively resolved; however, factors such as iron, inflammation and oxidative stress have been shown to exert an inhibitory effect on its expression [14]. Iron reduces hepcidin expression by interacting with transferrin receptors 1 and 2; this displaces the membrane protein HFE (believed to compete for biding with holo-transferrin), leading to stimulation of hepcidin production [22, 24]. Bone morphogenic protein (BMP6) also exerts an effect on hepcidin up-regulating release by interacting with the surface protein hemojuvelin (HJV) [22, 24]. Since neither BMP nor HJV sense iron directly, matriptase-2 [also known as transmembrane protease serine 6 (TMPRSS6)]-mediated cleavage of HJV is implicated in this pathway [14, 22]. As the majority of iron within the body is incorporated into hemoglobin, it is widely believed that an unidentified erythroid regulator must be present [14].
Hemochromatosis is caused by a series of mutations that effect multiple regulatory proteins at various points along the pathway of hepcidin and iron regulation due to mutations in the HFE and non-HFE (FPN, TFR, HJV) genes (Fig. 1).
Iron absorption and release in enterocytes and macrophages. Non-heme iron is taken up through the divalent metal transporter (DMT1) protein as Fe2+. Fe3+ is reduced to Fe2+ by duodenal cytochrome B (Dcytb). Heme iron is taken up through a heme transport protein and heme oxygenase. Once in the labile pool within the cell, Fe2+is compounded to the storage molecule ferritin. Iron is released from macrophages and enterocytes as Fe2+ through the transport protein ferroportin (FPN). Fe2+ is then oxidised to Fe3+ by the protein hephaestin and immediately compounded to transferrin. Factors affecting hepcidin release by the liver are the interaction between the HFE gene product and transferrin receptors 1 and 2 (TFR1 and TFR2), the interaction between bone morphogenic protein (BMP6) hemojuvelin (HJV) and the bone morphogenic protein receptor (BMP-R), and matriptase 2 (MT-2). Hepcidin in turn regulates FPN by triggering internalisation and degradation of FPN (adapted from Ganz [22]; Lawen and Lane [14])
Genetics and penetrance
The genetic bases for hemochromatosis can be divided principally into HFE gene mutations and non-HFE mutations [25]. The presence of non-HFE hemochromatosis was elucidated by Zarrilli et al. [26] when a purely clinical diagnosis of hemochromatosis yielded a higher incidence rate when compared with the genetic diagnosis based on the HFE genotype. Whilst not as common as HFE mutations, they show an increased proportion in non-Northern European populations and are therefore of importance in Asia-Pacific populations.
Hemochromatosis is then further subdivided into four overall types. Types I–III are linked to altered or reduced expression of hepcidin [11, 25, 27], whereas type IV results from reduced iron export [1, 28]. Mutations in HFE, HJV, HAMP, TFR2 and SLC40A1 have been linked to the various types of hemochromatosis [11, 25, 29], each displaying different onsets, severities and prevalences [2, 4, 9, 25, 27, 29–33] (Table 1).
HFE-associated hereditary hemochromatosis
The C282Y substitution resulting from a missense mutation in HFE is the most common cause of hereditary hemochromatosis in Caucasian populations [25, 30], with up to 90 % of hemochromatosis cases being associated with homozygosity for the mutation [9, 25, 30, 34]. However, there is significant variance in C282Y incidence with ethnic diversity [1, 29, 35, 36]. The highest allelic prevalence is in northern European populations (6 %), within which those of Celtic origin show an increased prevalence at 10–12.5 [9, 37]. Looking at non-European populations, a study by Adams et al. [38] found that whilst 0.44–0.68 % of Caucasians were homozygous for C282Y [36], the prevalence was only 0.11, 0.027, 0.014, 0.012 and 0.000039 % in Native American, Hispanic, Black, Pacific Islander and Asian populations respectively.
The penetrance of disease in the mutation is relatively low [34, 37]; Pietrangelo [32] suggests that between 10 and 33 % of homozygous patients develop hereditary hemochromatosis. This implicates other genetic and non-genetic factors in the disease [31]. To this end an Italian study found an I148 M mutation of the PNPLA3 gene increases both the risk and severity of hemochromatosis in the presence of C282Y homozygosity [33]. A study of 31,192 northern Europeans by Allen et al. [36] found a difference in disease between sexes, with 28.5 % of males and 1.2 % of females developing hereditary hemochromatosis by age 65. Mouse models suggest that this discrepancy is to do with females having naturally higher hepcidin levels, and not just menstruational loss of iron [9]. Furthermore, a large proportion of C282Y homozygotes remain asymptomatic despite elevated ferritin and transferrin levels [37].
Other mutations of HFE are known to exist, mainly S65C and H63D, but these do not by themselves lead to significant iron overload. H63D is of greater clinical interest [25, 27, 30, 32]. Having a prevalence of 10–20 % in all non-Asian populations [9, 25, 32, 39], H63D is rarely pathological on its own. It usually requires compound heterozygosity to cause symptomatic disease [9, 30]. Due to the increased prevalence of C282Y, compound heterozygotes are usually C282Y/H63D [9]. Whilst the prevalence is still higher, at 2 % of the Caucasian population, only 0.5–2 % of these people actually develop clinical disease [9, 34]. The proportion of hemochromatosis patients with a compound H63D heterozygosity varies between countries: in northern Europe 2.4–5 % [9, 34, 36], 7.5–10 % in Spain and 23.4 % in Brazil [29, 39]. Similar to C282Y homozygotes, a higher proportion of compound heterozygotes have elevated biochemical penetrance of iron overload without being clinically symptomatic [9]. S65C is a rare mutation, also associated with compound C282Y heterozygosity [32], and thus it is of little clinical importance.
Non-HFE hemochromatosis
Type IIa/b, or juvenile hemochromatosis, is the most severe form. Type IIa is due to mutations in HJV leading to truncated protein products or altered binding sites. This results in ineffective surface translocation, or binding to BMP, and thus reduced activation of hepcidin [40]. Type IIb is due to mutations of hepcidin [1, 29, 40] affecting the cysteine fingers or producing a null gene product [40]. Both sets of mutations result in a markedly reduced functioning of hepcidin, with an earlier clinical onset. Similar to HFE-hemochromatosis both are autosomal recessive, but unlike HFE-hemochromatosis there is no gender predominance in disease in type II. Type IIa is the more common, with G320 V mutations occurring in European and Brazilian cohorts [25, 29].
Type III hemochromatosis is a mutation of TFR2 [1, 2, 25, 29, 30, 41]. Whilst the function of TFR2 is not completely understood, it is believed to bind to sense transferrin in hepatocytes by binding to HFE [40]. Because of this, dysfunction of TFR2 results in reduced hepcidin production [40]. This type is most prevalent in Japan and Italy, but has also been seen in Brazil, France, Thailand and Portugal [1, 11, 25, 30]. Most cases are very rare compound heterozygotes, with over 30 mutations being seen in around 50 families [25, 30, 41]. A synonymous polymorphism, p.A617A, was found in seven compound heterozygotes in Brazil [29]. Whilst most common in Italy [1] in Japan the I238 M mutation occurs with a 7 % allele frequency, making it the leading cause of hemochromatosis in the region [25]. The clinical onset of type III is generally similar to HFE-hemochromatosis [1], but cases of juvenile-onset type III hemochromatosis have been documented [41].
Type IV hemochromatosis is the only autosomal dominant form of the disease [1, 40], interfering with FPN function. It affects the release of iron stores from Kupffer cells in the liver [28]. Whilst varied, the only mutation reported in multiple groups is a V162del missense mutation. Loss of FPN function reduces the cell’s ability to export iron, resulting in hyperferritinemia and iron sequestration in macrophages and enterocytes [1, 40]. This confusingly can result in iron deficiency anemia due to a reduced circulating iron and is associated with reduced end-organ damage and reduced need for venesection [28, 40]. However, similarly to the other non-HFE hemochromatoses, this form of the disease is associated with many sporadic mutations [1, 29]. There is also a rare form that is functional, but resistant to hepcidin inhibition [40]. This results in ‘atypical ferroportin disease’, which presents as typical hemochromatosis, with a build-up of iron in hepatocytes and other organs [1, 40].
Clinical expression
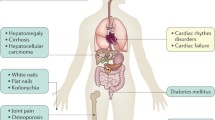
Iron deposition can occur in multiple tissues, resulting in a complex and variable clinical picture [9, 12]. However, it is worth noting that some organ systems are affected more readily than others, the heart, liver, pancreas, pituitary, skin and even joints are among these. These can result in hepatic fibrosis/cirrhosis, cardiomyopathy, impotence, gonadal atrophy, diabetes and arthritis [42, 43]. Interestingly, the arthritis in hemochromatosis primarily involves the 2nd and 3rd metacarpophalangeal joints and is due to calcium pyrophosphate deposition, not iron sequestration [42, 44]. The associated stigmata of chronic liver disease, pigmentation of the skin and diabetes form an unmistakable triad. However, owing to better screening it is much more likely that hemochromatosis patients will present with nonspecific signs: lethargy, arthralgia and weakness [42, 45]. Also noteworthy is that hepatomegaly is the most common finding on physical examination [32]. In Caucasians presenting with this picture, the index of suspicion for hemochromatosis must be high, and further investigations must be undertaken. Symptoms such as lethargy, weakness, skin pigmentation and hepatic fibrosis may regress with appropriate treatment [9, 46]. However, cardiomyopathy, cirrhosis and diabetes are irreversible.
Other lifestyle and environmental factors have been shown to impact disease progression in hemochromatosis. Male sex, alcohol consumption, hepatic steatosis from obesity and liver disease due to viral hepatitis have all been shown to increase the rate of disease progression [12, 47–49]. In contrast, female sex, regular consumption of tea, reduced gastric acidity and non-citrus fruit consumption have been shown to have protective effects [50].
Diagnosis
When a high clinical suspicion of hemochromatosis is present, biochemical studies form the basis for initial diagnosis (Fig. 2). If a patient is symptomatic, has hyperferritinemia or has a first degree relative with hemochromatosis further biochemical testing is indicated [3, 12]. To this end the transferrin saturation (TS), unsaturated iron binding capacity and serum ferritin (SF) are used in tandem [10]. Investigations for common causes affecting body iron levels, such as infection or inflammation, should also be carried out to rule out any confounding factors. In patients at risk of liver disease these avenues should be fully investigated and treated, especially viral and alcoholic hepatitis [12]. After careful consideration of all the aforementioned risk/complicating factors, TS elevated above 45 % with SF greater than 300 μg/l in males and 200 μg/l in females is a strong indication for HFE genotyping [3, 12]. For a detailed diagnostic algorithm, refer to Fig. 3.
Diagnostic algorithm. Persons of interest (individuals with asymptomatic hyperferritnaemia, the general population) undergo testing for transferrin saturation (TS) or unsaturated iron-binding capacity. If TS is lower than 45 % reassure and retest at a later point. Those with affected first degree family members and those with TS ≥45 % undergo genotype testing for HFE gene defects. Normal genotypes are counselled and considered for non-HFE hemochromatosis. C282Y homozygotes and C282Y/H63D compound heterozygotes are further evaluated with serum ferritin (SF), liver function tests (LFTs) and TS. If SF is ≤300 mg/l observe and retest in 1–2 years. If LFTs are abnormal and/or SF elevated above 1,000 mg/l then refer for liver biopsy. If biopsy shows no iron overload investigate and treat as appropriate. If SF is between 300 and 1,000 mg/l and LFTs are normal or if liver biopsy confirms iron overload then refer for phlebotomy. Reproduced with permission from Eijkelkamp et al. [55]
Genetic testing for the C282Y, H63D and S65C hemochromatosis mutations is readily available. However, the rarer genetic causes (discussed earlier) can only be tested for in a few specific centres [9]. Also note that only C282Y and H63D are of clinical relevance.
Liver biopsy is the most accurate determinant of fibrosis and cirrhosis, and it has great value in determining patient prognosis, especially in patients with SF over 1,000 μg/l or with other comorbid liver diseases [51]. Prior to the advent of non-invasive tests for hepatic fibrosis and testing for genetic markers, liver biopsy formed the backbone of hemochromatosis diagnosis. Hepatic iron distribution varies between the various subtypes of hemochromatosis and these patterns of stainable hepatic iron can be clearly elucidated following biopsy [52]. However, recently safer, less invasive methods, such as HFE gene testing, are favoured, for example, the Fibroscan® and magnetic resonance.
Since the move away from routine liver biopsy in diagnosis, T2*-weighted magnetic resonance imaging (MRI T2*) is generally used in its stead. The greater hepatic iron content in hemochromatosis is quantifiable in the high-intensity magnetic field used in MRI [9, 53].
Treatment and screening
Once diagnosed either surveillance or a treatment protocol must be undertaken. According to the consensus on treatment from the European Association for the Study of Liver (EASL) if the serum ferritin is within normal range, yearly follow-up is recommended [12]. If serum ferritin is elevated, treatment with venesection is recommended to bring serum ferritin levels down to maintenance levels [3, 12].
Venesection or phlebotomy is still the only widely accepted treatment for hemochromatosis. However, treatment with iron chelators and erythrocytophoresis has been recoded in the literature. Even though no randomised controlled trials have documented the efficacy of phlebotomy, it is known that treatment has beneficial effects on certain symptoms of disease. It works in two ways, first blood loss directly reduces the hemoglobin stores of iron; second, this induces erythropoiesis, which mobilises stored iron. Although highly variable, it has been reported that on average phlebotomy removes roughly 200–250 mg of iron per session [54]. This means depending on the patient’s iron status, the interval at which phlebotomy should be performed and the number of treatments required are highly variable. With recommendations permitting a reduction in interval to 2 weeks, what is most important in determining the treatment protocol is the SF maintenance level. Previous recommendations were to maintain SF below 50 μg/l. However, new literature suggests that this may lead to increased iron absorption in the gut, with indications that overzealous control of SF levels may have deleterious effects. Hence, at present the expert consensus is that an iron level between 50 and 100 μg/l with frequent monitoring is a better strategy [9, 11, 12]. There is insufficient evidence in the literature regarding clinical endpoints to treatment; however, as mentioned earlier the aim is to maintain serum ferritin within a certain range.
Weighing up the economic costs and health benefits of surveillance, population-level screening for HFE hemochromatosis is not recommended at present. Cascade screening of families with affected individuals is a much more favourable alternative [12]. When coupled with appropriate counselling regarding the pros and cons of testing and diagnosis, the current literature recommendations are to screen siblings and relatives of individuals with homozygosity for C282Y. The 25 % likelihood that immediate siblings are also homozygotes makes a strong case for this type of screening [12].
Conclusion and perspective
From the time of its first description and characterisation to the current understanding of its complex pathophysiology, the consensus opinion on hereditary hemochromatosis has changed considerably. At present it is understood that hemochromatosis results from various inherited defects, causing aberrations in molecules involved at different points in iron homeostasis. The different genetic causes vary greatly in prevalence both between and within populations. The incomplete penetrance of disease means diagnosis cannot be purely based on genetic screening. As such, currently diagnosis relies on a combination of imaging, biochemical iron and genetic studies.
Future directions of study will need to focus on the areas of diagnosis and treatment. With regard to diagnosis disease-modifying genes are promising as a fruitful avenue of research. So far a couple of these have been identified, one of which is GNPAT, first mentioned by Emond et al. in 2013 (abstract in Blood). However, the exact structure and function of GNPAT is currently not known. With regard to treatment randomised control trials into the efficacy of phlebotomy would be of considerable interest, particularly in relation to symptoms such as lethargy.
Due to the cost and logistics of population level screening, at present cascade screening of families of individuals affected with C282Y hemochromatosis is advised as these individuals are at markedly increased risk of iron overload disease. Studies into factors influencing disease progression in carriers of genetic mutations are required to further elucidate the reasons for incomplete penetrance in affected individuals.
References
Siddique A, Kowdley KV. Review article: the iron overload syndromes. Aliment Pharmacol Ther 2012;35(8):876–893. doi:10.1111/j.1365-2036.2012.05051.x
Zamani F, Bagheri Z, Bayat M, Fereshtehnejad SM, Basi A, Najmabadi H, et al. Iranian hereditary hemochromatosis patients: baseline characteristics, laboratory data and gene mutations. Med Sci Monit Int Med J Exp Clin Res 2012;18(10):CR622–CR629
Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Phys 2013;87(3):183–190
Kanwar P, Kowdley KV. Metal storage disorders: Wilson disease and hemochromatosis. Med Clin N Am 2014;98(1):87–102. doi:10.1016/j.mcna.2013.09.008
Guggenheim KY. Chlorosis: the rise and disappearance of a nutritional disease. J Nutr 1995;125(7):1822–1825
Trousseau A. Glycosurie, diabete sucre. Clinique medicale de l’Hotel-Dieu de Paris 1865;2:663–698
Von Recklinghausen F. Uber haemochromatose. Tageblatt Versammlung Dtsche Naturforscher Arzte Heidelberg 1889;62:324–325
Sheldon JH (1935) Haemochromatosis. Oxford University Press, Humphrey Milford, publisher to the University
Kanwar P, Kowdley KV. Diagnosis and treatment of hereditary hemochromatosis: an update. Exp Rev Gastroenterol Hepatol 2013;7(6):517–530. doi:10.1586/17474124.2013.816114
Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of 845G→A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 2002;359(9302):211–218. doi:10.1016/S0140-6736(02)07447-0
Wood MJ, Skoien R, Powell LW. The global burden of iron overload. Hepatol Int 2009;3(3):434–444. doi:10.1007/s12072-009-9144-z
European Association for the Study of the L. EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol 2010;53(1):3–22. doi:10.1016/j.jhep.2010.03.001
Fenton H. LXXIII.—Oxidation of tartaric acid in presence of iron. J Chem Soc Trans 1894;65:899–910
Lawen A, Lane DJ. Mammalian iron homeostasis in health and disease: uptake, storage, transport, and molecular mechanisms of action. Antioxid Redox Signal 2013;18(18):2473–2507. doi:10.1089/ars.2011.4271
Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc Lond Ser A Math Phys Sci 1934;147(861):332–351
Han O. Molecular mechanism of intestinal iron absorption. Metallomics 2011;3(2):103–109. doi:10.1039/c0mt00043d
Bourdon E, Kang D-K, Ghosh MC, Drake SK, Wey J, Levine RL, et al. The role of endogenous heme synthesis and degradation domain cysteines in cellular iron-dependent degradation of IRP2. Blood Cells Mol Dis 2003;31(2):247–255
Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997;388(6641):482–488
Greenberg GR, Wintrobe MM. A labile iron pool. J Biol Chem 1946;165(1):397–398
Bartnikas TB. Known and potential roles of transferrin in iron biology. Biometals 2012;25(4):677–686
Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim et Biophys Acta (BBA) Gen Subj 2009;1790(7):589–599
Ganz T. Systemic iron homeostasis. Physiol Rev 2013;93(4):1721–1741
Morgan E. Transferrin, biochemistry, physiology and clinical significance. Mol Asp Med 1981;4(1):1–123
Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim et Biophys Acta (BBA) Mol Cell Res 2012;1823(9):1434–1443
McDonald CJ, Wallace DF, Crawford DH, Subramaniam VN. Iron storage disease in Asia-Pacific populations: the importance of non-HFE mutations. J Gastroenterol Hepatol 2013;28(7):1087–1094. doi:10.1111/jgh.12222
Zarrilli F, Elce A, Scorza M, Giordano S, Amato F, Castaldo G. An update on laboratory diagnosis of liver inherited diseases. BioMed Res Int 2013;2013:697940. doi:10.1155/2013/697940
Bassett ML, Hickman PE, Dahlstrom JE. The changing role of liver biopsy in diagnosis and management of haemochromatosis. Pathology 2011;43(5):433–439. doi:10.1097/PAT.0b013e3283490e04
Brissot P. Hereditary hemochromatosis. Hematology 2013;18(6):370–371. doi:10.1179/1024533213Z.000000000222
Santos PC, Cancado RD, Pereira AC, Schettert IT, Soares RA, Pagliusi RA, et al. Hereditary hemochromatosis: mutations in genes involved in iron homeostasis in Brazilian patients. Blood Cells Mol Dis 2011;46(4):302–307. doi:10.1016/j.bcmd.2011.02.008
Radio FC, Majore S, Binni F, Valiante M, Ricerca BM, De Bernardo C, et al. TFR2-related hereditary hemochromatosis as a frequent cause of primary iron overload in patients from Central-Southern Italy. Blood Cells Mol Dis 2014;52(2):83–87
Fracanzani AL, Piperno A, Valenti L, Fraquelli M, Coletti S, Maraschi A, et al. Hemochromatosis in Italy in the last 30 years: role of genetic and acquired factors. Hepatology 2010;51(2):501–510. doi:10.1002/hep.23333
Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology 2010;139(2):393–408. doi:10.1053/j.gastro.2010.06.013 (408 e391–e392)
Dongiovanni P, Donati B, Fares R, Lombardi R, Mancina RM, Romeo S, et al. PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol 2013;19(41):6969–6978. doi:10.3748/wjg.v19.i41.6969
Sood R, Bakashi R, Hegade VS, Kelly SM. Diagnosis and management of hereditary haemochromatosis. Br J Gen Pract 2013;63(611):331–332. doi:10.3399/bjgp13X668410
Sikorska K, Romanowski T, Stalke P, Izycka-Swieszewska E, Bielawski KP. Iron overload and HFE gene mutations in Polish patients with liver cirrhosis. Hepatobiliary Pancreat Dis Int 2011;10(3):270–275
Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med 2008;358(3):221–230. doi:10.1056/NEJMoa073286
Powell LW, Dixon JL, Ramm GA, Purdie DM, Lincoln DJ, Anderson GJ, et al. Screening for hemochromatosis in asymptomatic subjects with or without a family history. Arch Intern Med 2006;166(3):294–301
Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 2005;352(17):1769–1778. doi:10.1056/NEJMoa041534
Castiella A, Zapata E, Otazua P, Zubiaurre L, Fernandez J. Non-HFE–related hemochromatosis: the role of genetic factors. Hepatology 2010;51(4):1473–1474
Camaschella C, Poggiali E. Rare types of genetic hemochromatosis. Acta Haematol 2009;122(2–3):140–145. doi:10.1159/000243798
Bardou-Jacquet E, Cunat S, Beaumont-Epinette MP, Kannengiesser C, Causse X, Sauvion S, et al. Variable age of onset and clinical severity in transferrin receptor 2 related haemochromatosis: novel observations. Br J Haematol 2013;162(2):278–281
Cadet E, Capron D, Perez AS, Crepin SN, Arlot S, Ducroix JP, et al. A targeted approach significantly increases the identification rate of patients with undiagnosed haemochromatosis. J Intern Med 2003;253(2):217–224
Bacon BR, Sadiq SA. Hereditary hemochromatosis: presentation and diagnosis in the 1990s. Am J Gastroenterol 1997;92(5):784–789
Timms AE, Sathananthan R, Bradbury L, Athanasou NA, Wordsworth BP, Brown MA. Genetic testing for haemochromatosis in patients with chondrocalcinosis. Ann Rheum Dis 2002;61(8):745–747
Swinkels DW, Aalbers N, Elving LD, Bleijenberg G, Swanink CM, van der Meer JW. Primary haemochromatosis: a missed cause of chronic fatigue syndrome? Neth J Med 2002;60(11):429–433
Falize L, Guillygomarc’h A, Perrin M, Laine F, Guyader D, Brissot P, et al. Reversibility of hepatic fibrosis in treated genetic hemochromatosis: a study of 36 cases. Hepatology 2006;44(2):472–477. doi:10.1002/hep.21260
Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS, American Association for the Study of Liver D. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the study of liver diseases. Hepatology 2011;54(1):328–343. doi:10.1002/hep.24330
Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW. A population-based study of the clinical expression of the hemochromatosis gene. N Engl J Med 1999;341(10):718–724. doi:10.1056/NEJM199909023411002
Adams PC, Agnew S. Alcoholism in hereditary hemochromatosis revisited: prevalence and clinical consequences among homozygous siblings. Hepatology 1996;23(4):724–727. doi:10.1002/hep.510230411
Hutchinson C, Geissler CA, Powell JJ, Bomford A. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut 2007;56(9):1291–1295. doi:10.1136/gut.2006.108613
Morrison ED, Brandhagen DJ, Phatak PD, Barton JC, Krawitt EL, El-Serag HB, et al. Serum ferritin level predicts advanced hepatic fibrosis among US patients with phenotypic hemochromatosis. Ann Intern Med 2003;138(8):627–633
Jehn M, Clark JM, Guallar E. Serum ferritin and risk of the metabolic syndrome in US adults. Diabetes Care 2004;27(10):2422–2428
ST Pierre TG, Clark PR, Chua-Anusorn W. Measurement and mapping of liver iron concentrations using magnetic resonance imaging. Ann N Y Acad Sci 2005;1054(1):379–385
Harrison SA, Bacon BR. Hereditary hemochromatosis: update for 2003. J Hepatol 2003;38:14–23
Eijkelkamp EJ, Yapp TR, Powell LW. HFE associated hereditary hemochromatosis. Can J Gastroenterol 2000;14:121–125
Compliance with ethical requirements and Conflict of interest
This article does not contain any studies with human or animal subjects. Dilum Ekanayake, Clinton Roddick and Lawrie Powell have nothing to disclose regarding conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ekanayake, D., Roddick, C. & Powell, L.W. Recent advances in hemochromatosis: a 2015 update. Hepatol Int 9, 174–182 (2015). https://doi.org/10.1007/s12072-015-9608-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-015-9608-2