Abstract
The purpose of this study was to examine the immunohistochemical expression of p53 and cytokeratin 19 (CK19) in normal oral mucosa (NOM) and oral squamous cell carcinoma (OSCC) and their association with histopathological differentiation grade. The secondary goal was to see if there was any correlation between p53 and CK19 expression in NOM and OSCC. A hospital-based retrospective analysis was conducted in which 40 NOM and 45 OSCC samples were acquired from archives and stained with mouse monoclonal antibodies p53 and CK19. For both the NOM and OSCC groups, the proportion of positively stained cells, staining intensity, and staining index were calculated. p53 immunoexpression revealed that 85% of positively stained cells in the NOM basal layer had a low staining index (mean ± SD 1.87 ± 0.34), whereas 66.7% of positively stained cells in the OSCC had a high staining index (mean ± SD 5.63 ± 3.02). When NOM and OSCC were compared, there was a statistically significant difference in staining intensity. However, despite a linear increase in the percentage of positive cells from well to poorly differentiated, the comparison between histopathological grades was non-significant. CK19 exhibited 18.5% positively stained cells in the NOM basal layer with a low staining index (mean ± SD 1.57 ± 0.53), whereas OSCC samples showed 4.44% immunopositivity with a high staining index. p53 is a marker of oral carcinogenesis independent of histological grade and CK19 expression. Further, CK19 is a marker of dysfunctional epithelial differentiation but lacks sensitivity and specificity; however, it demands further multicentric studies with a large sample size to draw definitive conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral cancer accounts for about 3% of all cancers worldwide [1]. More than 300,000 new cases of oral cancer are reported worldwide every year [2]. Oral squamous cell carcinoma (OSCC) accounts for the vast majority of these instances [3]. Despite this, the 5-year overall survival rate of OSCC patients has remained below 55% over the last decade due to local aggressiveness and high rates of metastasis [4]. The presence or absence of cervical lymph node metastasis has a significant impact on OSCC patients’ prognosis [5, 6]. Clinical stage, depth of invasion, and histologic grade of malignancy are all highly linked with nodal involvement [7, 8]. Despite having a significant impact on nodal involvement, all these parameters may not necessarily represent the metastatic ability of the cancer cells. As a result, identifying a biomarker of OSCC with significant metastatic potential might be clinically advantageous. In epithelial cells, cytokeratins (CKs) are the primary structural constituents of the cytoskeleton [9, 10]. Over 20 distinct CKs have been found, with CK19 being the smallest acidic type I CK family protein. CK19 has been found to be expressed in a variety of tumour tissues, including breast cancer, colon cancer, lung cancer, head and neck cancer (HNC), and hepatocellular carcinoma (HCC) [11,12,13,14]. However, the relevance and roles of CK19 appear to change between cancers. Previous research has indicated that CK19 plays a tumour-suppressive role in breast cancer but a tumour-promoting role in colon and liver cancers. Although immunoreactivity for CK19 has been detected in OSCC tissues, the rates of positive expression and its roles in this disease are controversial. Mutation of the p53 gene is one of the most common events in oral carcinogenesis. The accumulation of p53 protein has also been detected in premalignant lesions, especially oral leukoplakia with dysplasia. This suggests that p53 gene mutation may be an early step in the malignant transformation of oral dysplastic lesions. Such patients can be identified by p53 protein specific immunohistochemistry [15]. Survival rates of oral squamous cell carcinoma (OSCC) have not improved since decades, attributed to the complex multistep process of oral carcinogenesis specifically involving oncogenes and tumour suppressor genes [16,17,18]. Point mutations in the tumour suppressor gene (TP53) are seen in 35–67% of OSCC, and overexpression is associated with poor survival rates [19, 20]. Most of the mutations prolong the half life of p53 protein (6 h) which is amenable to immunohistochemical detection, whereas a few truncated deletions escape detection [21, 22]. Cytokeratin 19 (CK19) is believed to be one of the target proteins of TP53 gene, usually expressed in the basal layer of non keratinized stratified squamous epithelium [23,24,25,26]. CK19 shows variable expression in OSCC, ranging from 29 to 100% with a role in its progression [27,28,29,30,31]. Two studies concluded that downregulation of CK19 expression with the acquisition of an invasive phenotype in OSCC. There is single study in the literature comparing CK19 and p53 on OSCC cell lines, hypothesizing that wild type p53 inhibits CK19 expression.
Thus, the present study was a maiden attempt with the aim to evaluate immunohistochemical expression of p53 and CK19 in different histopathological grades of OSCC. Secondary objective was to study the correlation (if any) between p53 and CK19 in NOM and OSCC.
Materials and Methods
This was an institutional based retrospective study approved by institutional research and ethical review committee (reference number “MUHS/PG-T/E1/3882/2015”). Formalin fixed paraffin embedded 45 biopsy specimens of OSCC (group II) and 40 of NOM (group I) were retrieved from archives. The OSCC samples were graded by Bryne’s grading system. 4 μm sections were deparaffinized (560 C for 15 min) and incubated using mouse monoclonal antibody to p53 and CK19 (Biogenx) respectively. The results were interpreted as per the method adopted by Etemad -Moghadam et al. for p53 and CK19 separately (Table 1).
Immunohistochemical Analysis
From FFPE blocks of 45 OSCC specimens, 5 micrometre thick sections on poly L lysine coated slides were subjected to IHC analysis of p53 RTU (Ready To Use), Primary antihuman rabbit antibody, Leica Biosystems, Japan), and CK19 (RTU, Primary antihuman rabbit monoclonal antibody, Leika). Tissue sections were deparaffinized in xylene (twice), treated with a graded series of alcohol (100%, 95%, 85%, and 75% ethanol), and then incubated in phosphate-buffered saline (PBS, pH 7.4) for 5 min. Heat-induced antigen retrieval was done by immersion in 10 mM Tris-ethylene diamine tetra-acetic acid with pH 9 at 600 W in a pressure boiler until two whistles. Endogenous peroxidase was inactivated by 3% hydrogen peroxide for 10 min. The tissue sections were incubated with primary antibodies against p53 and CK19 for 40 min in humidifying chambers followed by incubation with a secondary polyclonal conjugate (Dako, Glostrup, Denmark) for 30 min. Lastly, tissue sections were treated with diaminobenzidine as a substrate chromogen and counterstained with hematoxylin. As negative controls, tissue sections were treated with PBS instead of the primary antibody. Skin sections and lung SCC were taken as positive controls for CK19 and p53 respectively. The slides were then mounted, observed, and evaluated using a research microscope (Nikon Eclipse Ni-U) using NIS Basic research software.
Immunohistochemical Evaluation
The positivity for p53 and CK19 was evaluated using a research microscope (Nikon Eclipse Ni-U). The expression was quantitatively assessed on five randomly selected fields under 400X by grid aided image analysis using NIS Basic research software. Positivity for p53 was observed in the nucleus of the cell and in cytoplasm of the basal layer for CK19. The percentage positivity for both markers was calculated by the number of positive cells per 1000 in the specimen.
Statistical Analysis
The analysis was performed using SPSS version 20.0 (IBM Corp.) software. The mean number of positive cells was compared in two groups using the t-test; staining intensity and staining index were compared using Fisher‘s exact test.
Results and Discussion
The histological findings of OSCC may range from one location to the next within the same tumour, necessitating a meticulous examination for an appropriate diagnosis. A precise histological grading of OSCC is critical because it provides an indication of the severity of the lesion in addition to therapeutic therapy and predicts the likely clinical course of the disease. Several histological grading schemes are in use to predict OSCC clinical behaviour. Despite the fact that biopsy is still the gold standard in diagnosis, no single histological grading system has been matched with its molecular behaviour till date to explain the severity of the lesion. Incisional biopsies can provide early hint on tumour behaviour with minimum invasiveness, preventing overtreatment. The samples in the present study showed male predominance, with the alveolobuccal complex (42.22%) constituting the predominant site in OSCC, which is in accordance with the previous literature (Table 2). The NOM group showed p53 immunopositivity in 85% of samples with expression confined to nuclei of the basal layer of the epithelium [mean ± standard deviation (SD) 16.00 ± 5.20] (Fig. 1). The Staining index was predominantly low (mean ± SD 1.87 ± 0.34) as compared to OSCC (mean ± SD 5.63 ± 3.02). Despite wild type p53 being hard to detect by immunohistochemistry due to its short half life (20 min), the positivity may be attributed to physiological stabilisation caused by UV radiation, hypoxia and viral proteins leading to genotoxic stress, an increased half-life and thus being amenable to detection [32, 33]. This explains the variable expression of p53 in NOM in different studies ranging from no expression to focal patchy positivity and 18.3–30% in other studies. In our study, OSCC showed 66.67% p53 immunopositivity in the nuclei of the basal and suprabasal layers of epithelium and in invasive carcinoma tissue (mean ± SD 45.57 ± 26.47). The immunopositivity in OSCC correlates with accumulated mutant p53 protein [34]. However, immunonegativity in the remaining OSCC samples (33.3%) does not certify the total absence of defects, as some deletion mutations produce non-detectable truncated proteins [35,36,37].
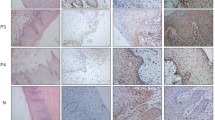
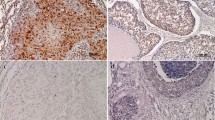
p53 and CK19 expression in NOM and WDSCC. (A) NOM showing parakeratinized stratified squamous epithelium overlying fibrous stroma (H&E, objective 10x). (B, C) p53 protein immunostaining of NOM restricted to nuclei basal layer of epithelium (Objective 10x, 40x), (D) CK19 protein staining cytoplasm of basal layer of epithelium (Objective 40x). (E) WDSCC showing islands of atypical epithelial cells (H&E, objective 10x), (F, G) p53 nuclear immunopositivity in invasive cancerous tissue (Obective10x, 40x) with high staining index. (H) CK19 staining invasive islands with high staining index (Objective 40x)
The percentage of overall p53 positive cells in OSCC samples was (66.67%) in our study. Montebugnoli et al. (57%) [38]; Yang et al. (62.5%) [40, 41]; Swaminathan et al. (65%) [42] found similar results for p53 positively stained cells in concordance with the present study. Hashmi AA et al. (66.1%) [43]; Verma et al. (66.6%) [37]; Kannan et al. 70%) [44], Bidaud P et al. (88.5%) [39]; and Kerdpon et al. (94%) [45] study results were contradictory to present study showing increased number of p53 positively stained cells .
In contrast to present study, some studies of p53 immunopositivity in OSCC such as ; Shiraki et al. (46%) [46] and Siegelmann-Danieli et al. (43%) [47] observed low expression for p53 positive cells. Wong et al. has suggested ubiquitin mediated rapid degradation of p53 by the E6 protein of human papilloma virus (HPV). The variable results of p53 expression in OSCC in different studies may be due to different techniques, types of antibodies, differences in ethnicity and risk factors associated with OSCC pathogenesis in heterogeneous populations.
In our study, within different grades of OSCC, the percentage of positive cells showed a linear increase from WDSCC to PDSCC (Table 3). The comparison of NOM with different grades of OSCC for staining intensity and staining index was highly significant (Table 4). However, all these parameters were statistically non-significant within the intra-group grade comparison of OSCC.
Similar to our study, a study by Siegelmann- Danieli [47], Abbas NF et al. [48] and Verma et al. [37] found non-significant association of p53 expression and tumor grading. However, Jain et al. (2008) [49] and Hashmi AA et al. (2018) [43] found a significant association between p53 over-expression with tumour grading and survival rates. This difference can be due to the small sample size in our study or p53 being an independent prognostic factor that is not dependent on histological grades [50]. For CK 19 immunoexpression (Table 1; Figs. 1 and 2) 17.5% samples of NOM showed immunopositivity restricted to the cytoplasm of the basal layer of epithelium [mean ± SD 18.29 ± 4.11]. 10% of samples showed moderate and 7.5% low staining intensity and low staining index [mean ± SD 1.57 ± 0.53] (Fig. 3). OSCC showed immunopositivity only in 2 (4.44%) samples of WDSCC [mean ± SD 41.00 ± 7.07] with high staining intensity and index (mean 6). A Statistical comparison between two groups with regard to staining index comparison was statistically significant (p 0.0019). Staining intensity comparison between two groups and within different histopathological grades of OSCC was not possible because of inadequate positive samples.
p53 and CK19 expression in MDSCC and PDSCC. (A, E) MDSCC (H&E, objective 10x), and PDSCC (H&E, objective 40x), showing sheets of malignant epithelial cells, (B-C, F-G) p53 staining nuclei of malignant epithelial cells with high staining index in MDSCC and PDSCC (objective 10x,40x), (D, H) CK19 immunonegativity in MDSCC and PDSCC (objective 40x)
Similar results were seen by Crowe et al. who observed consistent downregulation of CK19 expression in OSCC cell lines as compared to normal epithelium. Similarly, Kale AD et al., also found immunonegativity in all OSCC samples. The low (17.5%) expression of NOM in our study can be explained as most of our samples were of keratinized mucosa, and downregulation of CK19 is essential for terminal differentiation of keratinocytes. Immunonegativity in the majority of samples of OSCC in our study is justified as over-expression of CK19 decreases the invasive potential by diminishing the migratory capability or formalin fixation masking CK19 antigenic sites in most of the samples [7, 15].
However, most of the studies showed variable expression of CK19 in OSCC, such as Nie et al.(100%) [51] Zhong LP et al.(90.9%), Hamakawa et al.(66.7%) [52], Babiker AY et al. (58%) [53], Frohwitter G et al. (40.1%) [27], Fillies T et al. (40.6%) [28] and Vora HH et al. (29%) [54] and most studies showed a positive correlation of CK19 overexpression with increasing grade of OSCC and poor prognosis. The contrasting results in our study may be due to a different study population with heterogeneous risk factors, a small sample size, formalin fixation, which damages CK19 antigenic sites, different antibodies and antigen retrieval techniques used.
Conclusions
This study concluded that p53 is a marker of oral carcinogenesis independent of histopathological grade and CK19 expression. The switching of CK19 expression may serve as marker for invasion in OSCC; however, this criterion cannot be specified as only 4.44% of samples were immunopositive which indicates that CK19 is neither a reliable nor sensitive marker in oral carcinogenesis.
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- CK19:
-
cytokeratin 19
- OSCC:
-
oral squamous cell carcinoma
- NOM:
-
normal oral mucosa
- SD:
-
standard deviation
- WDSCC:
-
well differentiated squamous cell carcinoma
- MDSCC:
-
moderately differentiated squamous cell carcinoma
- PDSCC:
-
poorly differentiated squamous cell carcinoma
- IHC:
-
immunohistochemistry
References
Sharma G, Kamboj M, Narwal A et al (2022) Cytotoxic role of chlorogenic acid on oral squamous cell Carcinoma Cell line. Indian J Otolaryngol Head Neck Surg 74(Suppl 3):5773–5781. https://doi.org/10.1007/s12070-021-02395-1
Gupta B, Johnson NW, Kumar N (2016) Global epidemiology of head and neck cancers: a continuing challenge. Oncology 91:13–23
Johnson NW (1991) Orofacial neoplasms: global epidemiology, risk factors and recommendations for research. Int Dent J 41:365–375
Funk GF, Karnell LH, Robinson RA, Zen WK, Trask DK, Hoffman HT (2002) Presentation, treatment and outcome of oral cavity cancer: a national cancer data data base report. Head Neck 24:165–180
Platz H, Fries R, Fries R, Hudec M (1985) Retrospective DOSAK study on carcinomas of the oral cavity: results and consequences. J Maxillofac Surg 13:147–153
Snow GB, Patel P, Leemans CR, Tiwari R (1992) Management of cervical lymph nodes in patients with head and neck cancer. Eur Arch Otorhinolaryngol 249:187–194
Yamamoto E, Miyakawa A, Kohama G (1984) Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg 6:938–947
Almangush A, Mäkitie AA, Triantafyllou A, de Bree R, Strojan P, Rinaldo A, Hernandez-Prera JC, Suárez C, Kowalski LP, Ferlito A, Leivo I (2020) Staging and grading of oral squamous cell carcinoma: An update. Oral oncology. Aug 1;107:104799
Moll R, Franke WW, Schiller DL, Geiger B, Krepler R (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24
Moll R, Krepler R, Franke WW (1983) Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation 23:256–269
Wu YJ, Rheinwald JG (1981) A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell 25:627–635
Sugama Y, Kitamura S, Kawai T, Ohkubo A, Hasegawa S, Kuriyama T et al (1994) Clinical usefulness of CYFRA assay in diagnosing lung cancer: measurement of serum cytokeratin fragment. Jpn J Cancer Res 85:1178–1184
Wieskopf B, Demangeat C, Purohit A, Strenger R, Gries P, Kreisman H et al (1995) Cyfa21- 1 as a biologic marker of non-small cell lung cancer. Chest 108:163–169
Takahashi H, Kurishima K, Ishikawa H (2010) Optimal cutoff points of CYFRA21-1 for survival prediction in non‐small cell lung cancer patients based on running statistical analysis. Anticancer Res 30:3833–3837
Khanna R, Vidhyarthi AK, Khanna S, Singh S, Singh UC (2012) Expression of p53 protein in Leukoplakia and oral squamous cell carcinoma. World J Surg Med Radiation Oncol 1:16
Hamakawa H, Bao Y, Takarada M, Fukuzumi M, Tanioka H (1998) Cytokeratin expression in squamous cell carcinoma of the lung and oral cavity: an immunohistochemical study with possible clinical relevance. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:438–443
Nylander K, Dabelsteen E, Hall PA (2000) The p53 molecule and its prognostic role in squamous cell carcinomas of the head and neck. J Oral Pathol Med 29:413–425
Abrahao AC, Bonelli BV, Nunes FD, Dias EP, Cabral MG (2011) Immunohistochemical expression of p53, p16 and hTERT in oral squamous cell carcinoma and potentially malignant disorders. Braz Oral Res 25:34–41
Li L, Fukumoto M, Liu D (2013) Prognostic significance of p53 immunoexpression in the survival of oral squamous cell carcinoma patients treated with surgery and neoadjuvant chemotherapy. Oncol Lett 6(1):1611–1615
Cui XS, Donehower LA (2000 Dec) Differential gene expression in mouse mammary adenocarcinomas in the presence and absence of wild type p53. Oncogene 19:5988–5996
Kale AD, Mane DR, Babji D, Gupta K (2012) Establishment of field change by expression of cytokeratins 8/18, 19, and MMP-9 in an apparently normal oral mucosa adjacent to squamous cell carcinoma: a immunohistochemical study. J Oral Maxillofac Pathol 16:10–15
Safadi RA, Musleh AS, Al-Khateeb TH, Hamasha AA (2010) Analysis of immunohistochemical expression of k19 in oral epithelial dysplasia and oral squamous cell carcinoma using color deconvolution-image analysis method. Head Neck Pathol 4:282–289
Rao RS, Patil S, Ganavi BS Oral cytokeratins in health and disease. J Contemp Dent Pract. 2014 Jan 1;15(1):127 – 36
Lindberg K, Rheinwald JG (1989) Suprabasal 40 kd keratin (K19) expression as an immunohistologic marker of premalignancy in oral epithelium. Am J Pathol 134:89
Babiker AY, Rahmani AH, Abdalaziz MS, Albutti A, Aly SM, Ahmed HG (2014) Expressional analysis of p16 and cytokeratin19 protein in the genesis of oral squamous cell carcinoma patients. Int J Clin Exp Med 7:1524–1530
Vora HH, Shah NG, Patel DD, Trivedi TI, Chikhlikar PR (2003) Prognostic significance of biomarkers in squamous cell carcinoma of the tongue: multivariate analysis. J Surg Oncol 82:34–50
Frohwitter G, Buerger H, Van Diest PJ, Korsching E, Kleinheinz J, Fillies T (2016) Cytokeratin and protein expression patterns in squamous cell carcinoma of the oral cavity provide evidence for two distinct pathogenetic pathways. Oncol Lett 12:107–113
Fillies T, Jogschies M, Kleinheinz J, Brandt B, Joos U, Buerger H (2007) Cytokeratin alteration in oral leukoplakia and oral squamous cell carcinoma. Oncol Rep 18:639–643
Crowe DL, Milo GE, Shuler CE (1999) Keratin 19 downregulation by oral squamous cell carcinoma lines increases invasive potential. J Dent Res 78:1256–1263
Molès JP, Schiller JT, Tesniere A, Leigh IM, Guilhou JJ, Basset-Séguin N (1994) Analysis of HPV16 E6 and mutant p53-transfected keratinocytes in reconstituted epidermis suggests that wild-type p53 inhibits cytokeratin 19 expression. J Cell Sc 107:435–441
Soussi T (2000) The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann N Y Acad Sci 910:121–139
Oliveira LR, Ribeiro-Silva A (2011) Prognostic significance of immunohistochemical biomarkers in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 40:298–307
Bhargava A, Saigal S, Chalishazar M (2010) Histopathological grading systems in oral squamous cell carcinoma: a review. J Int Oral Health 2:1–10
Etemad-Moghadam S, Khalili M, Tirgary F, Alaeddin M (2009) Evaluation of myofibroblasts in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol 38:625–643
Kumar Shukla N, Deo SS, Jakhetiya A, Manjunath NM, Sreenivas V, Thulkar S et al (2018) Clinical spectrum, treatment and relapse patterns in 353 patients with squamous cell carcinoma of the Alveobuccal Complex treated with a curative intent: a retrospective study. J Maxillofac Oral Surg 17:24–31
Ries JC, Schreiner D, Steininger H, Girod SC (1998) p53 mutation and detection of p53 protein expression in oral leukoplakia and oral squamous cell carcinoma. Anticancer Res 18(3B):2031–2036
Verma R, Singh A, Jaiswal R, Chandra A, Verma R, Tak J (2014) Association of Ki 67 antigen and p53 protein at invasive tumor front of oral squamous cell carcinoma. Indian J Pathol Microbiol 57:553–557
Montebugnoli L, Felicetti L, Gissi DB, Cervellati F, Servidio D, Marchetti C et al (2008) Predictive role of p53 protein as a single marker or associated to Ki67 antigen in oral carcinogenesis. Open Dent J 2:24–29
Bidaud P, Chasle J, Sichel F, Rousseau S, Petit P, Pottier D et al (2010) Expression of p53 family members and CD44 in oral squamous cell carcinoma (OSCC) in relation to tumorigenesis. Histol Histopathol 25:331–339
Yang L, Wang Y, Guo L, Wang L, Chen W, Shi B (2015) The expression and correlation of iNOS and p53 in oral squamous cell carcinoma. Biomed Res Int. ; 2015: 637853
Cuevas Gonzalez JC, Gaitan Cepeda LA, Borges Yanez SA, Cornejo AD, Mori Estevez AD, Huerta ER (2016) p53 and p16 in oral epithelial dysplasia and oral squamous cell carcinoma: a study of 208 cases. Indian J Pathol Microbiol 59(2):153–158
Swaminathan U, Joshua E, Rao UK, Ranganathan K (2012) Expression of p53 and cyclin D1 in oral squamous cell carcinoma and normal mucosa: an immunohistochemical study. J Oral Maxillofac Pathol 16:172–177
Hashmi AA, Hussain ZF, Hashmi SK, Irfan M, Khan EY, Faridi N et al (2018) Immunohistochemical over expression of p53 in head and neck squamous cell carcinoma: clinical and prognostic significance. BMC Res Notes 11:433
Kannan S, Chandran GJ, Pillai KR, Mathew B, Sujathan K, Nalinakumary KR et al (1996) Expression of p53 in leukoplakia and squamous cell carcinoma of the oral mucosa: correlation with expression of Ki67. Clin Mol Pathol 49:170–175
Kerdpon D, Rich AM, Reade PC (1997) Expression of p53 in oral mucosal hyperplasia, dysplasia and squamous cell carcinoma. Oral Dis 3:86–92
Shiraki M, Odajima T, Ikeda T, Sasaki A, Satoh M, Yamaguchi A et al (2005) Combined expression of p53, cyclin D1 and epidermal growth factor receptor improves estimation of prognosis in curatively resected oral cancer. Mod Pathol 18:1482–1489
Siegelmann-Danieli N, Ben-Izhack O, Hanlon A, Ridge JA, Stein ME, Khandelwal V et al (2005) P53 alteration in oral tongue cancer is not significantly associated with age at diagnosis or tobacco exposure. Tumori 91:346–350
Abbas NF, El-Sharkawy SL, Abbas EA, El-Shaer MA (2007) Immunohistochemical study of p53 and angiogenesis in benign and preneoplastic oral lesions and oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:385–390
Jain A, Maheshwari V, Mehdi G, Alam K, Sharma SC (2008) Diagnostic and prognostic significance of p53 protein expression in squamous cell lesions of the oral cavity. Int J Otorhinolaryngol 7:45–49
De Oliveira LR, Ribeiro-Silva A, Zucoloto S (2007) Prognostic impact of p53 and p63 immunoexpression in oral squamous cell carcinoma. J Oral Pathol Med 36(4):191–197
Nie MH, Zhong L, Zeng GM, Li BQ (2002) The changes of cytokeratin 19 during oral carcinogenesis. Chin J Stomatol 37:187–191
Hamakawa H, Bao Y, Takarada M, Fukuzumi M, Tanioka H (1998) Cytokeratin expression in squamous cell carcinoma of the lung and oral cavity: an immunohistochemical study with possible clinical relevance. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:438–443
Babiker AY, Rahmani AH, Abdalaziz MS, Albutti A, Aly SM, Ahmed HG (2014) Expressional analysis of p16 and cytokeratin19 protein in the genesis of oral squamous cell carcinoma patients. Int J Clin Exp Med 7:1524–1530
Vora HH, Shah NG, Patel DD, Trivedi TI, Chikhlikar PR (2003) Prognostic significance of biomarkers in squamous cell carcinoma of the tongue: multivariate analysis. J Surg Oncol 82:34–50
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Institutional research and ethical review committee (reference number “MUHS/PG-T/E1/3882/2015”).
Informed consent
For this type of study formal consent is not required.
Consent for Publication
For this type of study consent for publication is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, H., Hazarey, V., Sharma, G. et al. p53, Cytokeratin 19 Expression in Oral Squamous Cell Carcinoma and Correlation with Histopathologic Grading: An Immunohistochemical Study. Indian J Otolaryngol Head Neck Surg 76, 103–111 (2024). https://doi.org/10.1007/s12070-023-04092-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-023-04092-7