Abstract
Malignant otitis externa (MOE) is a rare and fatal condition affecting temporal bone. It is also known as skull base osteomyelitis and is a rapidly progressive condition. This retrospective study evaluates the clinical, haematological, microbiological profile and management of malignant otitis externa in a tertiary care hospital and literature review. A retrospective review of 79 patients diagnosed with Malignant Otitis Externa from January 2015 to June 2021 was analyzed. History and Clinical findings, Imaging, Bacteriology, Random blood sugar on admission, Erythrocyte Sedimentation rate, HbA1C level, Biopsy of the granulation tissue from Externa auditory canal, cranial nerve involvement, duration of hospital stay, and treatment outcomes were analyzed. Out of 79 patients, otorrhea, otalgia, EAC oedema, and granulation were the most common findings. Facial nerve paralysis was found in 20 patients (25.3%) and multiple cranial nerve paralysis in 5 patients (6.3%). Uncontrolled diabetes mellitus and older age have increased duration of hospital stay, while cranial nerve paralysis did not affect this duration. Six different microorganisms were isolated. Pseudomonas aeruginosa was the most common organism cultured. Ciprofloxacin resistance was detected in 79% of cases. Amikacin, Cefaperazone-Sulbactam, and Piperacillin were the most sensitive antibiotics for gram negative organisms in our study. This study reviews the current microbiological profile and shows the need for higher-end antibiotics to treat MOE in present times. Early diagnosis, aggressive control of diabetes mellitus, and long duration culture-sensitive antibiotic therapy with regular monitoring are essential to reducing morbidity and mortality due to MOE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant Otitis Externa (MOE) is an infective inflammatory condition affecting the external auditory meatus, temporal bone, and skull base. It’s a rare and fatal condition [1]. Chandler coined the term malignant in 1968, and the condition was first reported by Toulmouche in 1838 [2, 3]. This condition is caused most commonly by Pseudomonas aeruginosa. Staphylococcus aureus, Proteus mirabilis, and fungi like aspergillus and candida species can also be the causative agent [4]. The disease begins as an acute otitis externa and then spreads through the fissures of Santorini into the skull base and jugular foramina [5], causing osteomyelitis and cranial nerve palsies [1]. It commonly affects the elderly patients with diabetes mellitus though immunocompromised states such as HIV infection and chemotherapy are also susceptible to this condition. The clinical manifestation includes severe otalgia, otorrhea, and headache; as the condition progresses, patients can present with complications such as cranial nerve palsies, sinus thrombophlebitis, meningitis, and death [6]. The facial nerve is the commonly involved cranial nerve at the level of the stylomastoid foramen. The diagnosis is a combination of clinical features such as exudates, granulation at the bony-cartilaginous junction of the external ear canal, tragal tenderness, elevated erythrocyte sedimentation rate with positive bone scan (Technetium-99), and Computed tomography showing opacification of the external ear and mastoid with bony erosion, osteomyelitis of skull base. It can also affect the soft tissue surrounding the external auditory canal [1]. Bone scintigraphy is mainly helpful in monitoring the condition than in diagnosis. Gallium-67 is used for this purpose [7]. Treatment includes systemic anti-Pseudomonal therapy for about six weeks or more, along with topical treatment. Other modalities that play a minor role are surgical debridement, hyperbaric oxygen therapy, and antifungal used in primary treatment failure [4]. The most common cause attributed to the development of MOE is uncontrolled diabetes mellitus. This study evaluates the clinical, radiological, haematological, microbiological profile, and management of MOE, analyzing the current antibiotic sensitivity pattern in a tertiary care hospital in South India.
Materials and Methods
A retrospective review of all the patients presenting with MOE between January 2015 and December 2020 was studied. Seventy-nine patients were included in the study. The following details were collected from all the patients
-
1.
History and Clinical findings.
-
2.
Radiological findings.
-
3.
Microbiological findings.
-
4.
Random blood sugar testing on admission.
-
5.
Erythrocyte Sedimentation rate.
-
6.
HbA1C level.
-
7.
Biopsy of the granulation tissue from EAC.
Demographic data, cranial nerve involvement, duration of hospital stay, and treatment outcomes were reviewed. This study was performed in line with the principles of the Declaration of Helsinki. This research study was conducted retrospectively from data obtained for clinical purposes. Waiver of ethical approval was granted from the institutional review board of our institute.
All the patients were started on empirical systemic anti-pseudomonal therapy and changed to appropriate antimicrobial based on culture and sensitivity. Local treatment with ciprofloxacin/2% acetic acid ear drops and appropriate analgesics were used. All the patients were treated for 6–8 weeks. Diabetes mellitus was controlled with insulin and oral hypoglycemics after consultation with physicians. Criteria for discharge were falling ESR to normal range, absence of pain, and normal blood glucose levels. Statistical analysis was done using software SPSS version 26. Data were expressed as mean, Standard deviation, and percentage with p < 0.05 was considered statistically significant.
Results
a. History
Out of 79 patients studied, 70 were male (88.6%). The mean age was 66.30 ± 10.29 (range 50-86) years. Elderly patients (age > 60 years) had a statistically significant longer duration of hospital stay (p-0.02).
All the patients had otorrhea and otalgia (100%) at presentation. Headache was present in 61 patients (77.2%).
All the patients had diabetes mellitus (100%), and in addition, 25 patients (31.6%) had other comorbidities. Ten out of these 25 patients had more than two comorbidities. Hypertension was the most common comorbidity after diabetes mellitus.
b. Clinical Finding
The distribution of various clinical findings as shown in Table 1.
Grade 6 (House Brackmann grading) facial nerve paralysis was present in 15/20 patients at presentation, and grade 3 facial nerve palsy was noted in 5/20 patients. Multiple cranial nerves involved were glossopharyngeal, vagus, and hypoglossal nerves. The mean duration of hospital stay in patients with cranial nerve paralysis was 9.5 days, while 11.4 days in patients without paralysis. There was no significant difference (p-0.45) between the two groups.
Imaging
High-resolution computed tomography of the temporal bone was done in all patients, showing soft tissue density in the external ear canal in all the patients (100%). Erosion of anterior wall of external ear canal was noted in 52 patients (66%), involvement of temporomandibular joint was noted in 20 patients (25.3%), mastoid and middle ear involvement in 35 patients (44.3%), petrous apex involvement in 4 patients (5%) and clivus involvement in 2 patients (2.5%). Scintigraphy was done with technetium-99 which showed increased osteoblastic activity in 52 patients (65.8%).
Microbiology
Six different microorganisms were isolated from 79 patients (Table 2). No growth was detected in 10 patients(12.6%). Antibacterial susceptibility testing was done by Kirby–Bauer disc diffusion method, and interpretation of the sensitivity pattern was made according to Clinical & Laboratory Standards Institute guidelines (Table 3).
a. Pseudomonas Aeruginosa
For Amikacin and Cefaperazone- Sulbactam antibiotics, 90% of isolates were sensitive, followed by Piperacillin, to which 78% of isolates were sensitive. Resistance to Quinolones (Ciprofloxacin) was noted in 79% of patients.
b. Staphylococcus Aureus
All the MRSA isolates were sensitive to Vancomycin. About 87.5% of isolates were susceptible to Cloxacillin and Clindamycin each.
c. Other Organisms
Klebsiella pneumoniae was sensitive to Cefaperazone- Sulbactam and Amikacin in 90% and 79% of the isolates, respectively. Escherichia coli was susceptible to Cefaperazone- Sulbactam, Gentamicin, and Piperacillin predominantly.
4. The mean random blood glucose of all the 79 patients at the time of admission was 226.70 ± 123.79 mg/dl (range 73–460 mg/dl). The mean blood glucose level at the time of discharge was 110 ± 45.6mg/dl (range 60–160mg/dl).
5. The mean ESR was found to be 60 ± 23mm/h (range 40–92mm/h).
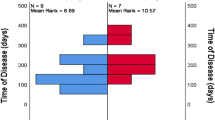
6. The glycemic control was measured using HbA1c. The mean HbA1c was 10.4 ± 4.4% (range 5.5%–17.4%). Based on HbA1c, patients were divided into ≤ 6 HbA1c and > 6 HbA1c (Table 4).
7. Biopsy of the granulation in the external auditory canal was done in 68 patients who had granulation tissue in the external auditory canal to rule out malignancy.
Discussion
Malignant Otitis Externa (MOE) is an infective inflammatory condition that commonly affects elderly male patients, and also older patients are at increased risk for disease-specific mortality [4]. The mean age of patients in our study was 66.30 ± 10.29 years, and older patients (> 60 years) had a longer hospital stay than young patients. The condition is characterized by otalgia, otorrhea, external auditory canal oedema, granulation, and microabscess (on surgery), which are considered obligatory criteria in diagnosing this condition [8]. In our study, all the patients had otalgia and otorrhea(100%). Headache was the next common symptom present in 58 patients (73.4%). Trismus was present in 13 patients (16.4%) due to the involvement of the temporomandibular joint following anterior canal wall erosion.
Facial nerve paralysis was present in 20 patients (25.3%). This finding was similar to the studies conducted by Franco-Vidal et al., who reported 20% of facial nerve involvement, and kaya et al., who had 20% with grade 6 paralysis and 8% with grade 3 paralysis [9, 10]. The facial nerve is the most common cranial to be involved by MOE at the level of stylomastoid foramen due to proximity to the external ear canal. This was followed by the involvement of lower cranial nerves like glossopharyngeal, vagus, spinal accessory, and hypoglossal nerve when the inflammation extends to the jugular foramen and skull base. Multiple cranial nerve paralysis was involved in 5 patients (6.3%) in our study. In their studies, Mani et al. and Sudry et al. found no significant difference in survival in MOE patients with facial nerve paralysis [12]. Lee et al. and Franco-Vidal et al. showed that facial paralysis in MOE patients significantly affects survival [6, 9]. None of the patients had mortality during the hospital stay or follow-up in 1 year in our study.
Diabetes mellitus is the most important comorbidity associated with this condition. But any condition which causes immunosuppression like HIV/AIDS, chemotherapy, post-transplantation can predispose to MOE [13, 14]. diabetes causes endarteritis, microangiopathy, and impairment of phagocytosis, along with the ability of Pseudomonas to cause vasculitis and vessel wall necrosis underlies the pathology [3]. In our study, all the patients had a history of Diabetes mellitus except one, who was incidentally diagnosed on admission. HbA1c plays a vital role in determining treatment duration, as adequate glycemic control forms an important aspect of management. The mean duration of hospital stay was significantly high when HbA1c > 6, which indicates a poor response to antibiotics in these patients and increased time required to achieve glycemic control before discharge.
Pseudomonas aeruginosa was the predominant organism isolated, followed by Staphylococcus aureus. Pseudomonas aeruginosa is a gram negative obligate aerobe. It colonizes EAC in moist conditions or after trauma. It generally causes acute otitis externa, but MOE develops only in patients with impaired host defences. The absence of any microorganism in ten patients was probably due to the use of topical/oral antibiotic medications before getting an ear swab done.
Ciprofloxacin is the most widely used drug to treat MOE due to the least side effect profile, high tolerability, and better cartilage penetration. As seen in previous studies, Pseudomonas started developing resistance to ciprofloxacin due to the widespread use of fluoroquinolones both in systemic therapy and topical ear drops. Berenholz et al. showed 33% resistance of Pseudomonas to ciprofloxacin in MOE [15]. In literature, resistance to ciprofloxacin by Pseudomonas was found to be 3%–50% [15,16,17]. In our study, resistance to ciprofloxacin by Pseudomonas was 79%. This shows a gradual rise in the resistance over time. This can be prevented by avoiding the use of quinolones for benign otitis externa in an outpatient setting. Pseudomonas, Klebsiella pneumoniae, and E. coli were predominantly sensitive to Amikacin, Cefaperazone- Sulbactam, and Piperacillin. This shows how antibiotic resistance has pushed the need to use higher-end antibiotics to treat this condition. MRSA organism was sensitive to Vancomycin in all the patients.
All the patients in our study were started on empirical high dose ciprofloxacin and topical ciprofloxacin after getting an ear swab done. Antibiotic was changed after three days following the culture sensitivity report. Sixty patients (75.9%) received Cefaperazone-Sulbactam, and five patients(6.3%) received high dose ciprofloxacin, and 14 patients(17.7%) received Ceftazidime. Shavit et al. reported that 73% of patients received Ceftazidime and the remaining high dose quinolones in his study[4]. Though Amikacin was sensitive in many cases, it was avoided due to its toxicity profile.
Quinolones have allowed effective outpatient treatment because of oral availability and reduced the duration of hospital stay [18]. All the patients were discharged when there was symptomatic improvement, ESR reached a normal level, and adequate glycemic control was attained. Patients were discharged on oral ciprofloxacin (high dose) for a total period of 6 weeks of antibiotics because of excellent bioavailability, good cartilage penetration, and fewer side effects. Only three patients (3.7%) in our study had fungal infections for which Voriconazole was used for treatment.
Recurrence is common in MOE. It has been reported to be 15–20%, so at least six months of follow-up is needed [19]. In our study, follow-up was done for 1 year, and only two patients had disease relapse who were treated with eight weeks of culture-sensitive antibiotic—none of the patients required any surgical intervention except for Biopsy of the granulation to rule out malignancy. Mortality from MOE has been reported to be 0%–15% [20]. But none of the patients had mortality in our study.
According to our study, uncontrolled diabetes mellitus and older age have increased hospital stay duration, while cranial nerve paralysis did not affect this duration. Ciprofloxacin resistance was 79% which is higher than what was reported in previous studies (3%–50%). Amikacin, Cefaperazone-sulbactam, and Piperacillin are antibiotics that can be used as an alternative to Ceftazidime and Ciprofloxacin-resistant cases. Though highly sensitive for Pseudomonas aeruginosa, Amikacin must be reserved when the organism is resistant to the other two antibiotics.
Conclusion
Malignant otitis externa is a rapidly progressive, invasive inflammatory condition associated with high morbidity and mortality. Antibiotic sensitivity has changed in present times to treat MOE. Aggressive control of diabetes mellitus and long-term culture-sensitive antibiotic therapy with regular monitoring is essential for the complete treatment of this condition.
Data Availability
All data and materials as well as software application or custom code support their published claims and comply with field standards.
References
Karaman E, Yilmaz M, Ibrahimov M, Haciyev Y, Enver O (2012) Malignant Otitis Externa. J Craniofac Surg 23(6):1748–1751
Bhandary S, Karki P, Sinha BK (2002) Malignant otitis externa: a review. Pac Health Dialog 9(1):64–67
Chandler JR (1968) Malignant external otitis. Laryngoscope 78(8):1257–1294
Stern Shavit S, Soudry E, Hamzany Y, Nageris B (2016) Malignant external otitis: Factors predicting patient outcomes. Am J Otolaryngol 37(5):425–430
Nadol JB Jr (1980) Histopathology of Pseudomonas osteomyelitis of the temporal bone starting as malignant external otitis. Am J Otolaryngol 1:359–371
Lee SK, Lee SA, Seon SW, Jung JH, Lee JD, Choi JY et al (2017) Analysis of prognostic factors in malignant external otitis. Clin Exp Otorhinolaryngol 10(3):228–235
Sreepada GS, Kwartler JA (2003) Skull base osteomyelitis secondary to malignant otitis externa. Curr Opin Otolaryngol Head Neck Surg 11:316–323
Cohen D, Friedman P (1987) The diagnostic criteria of malignant external otitis. J Laryngol Otol 101:216–221
Franco-Vidal V, Blanchet H, Bebear C, Dutronc H, Darrouzet V (2007) Necrotizing external otitis: A report of 46 cases. Otol Neurotol 28:771–773
Kaya İ, Sezgin B, Eraslan S, Öztürk K, Göde S, Bilgen C, Kirazlı T (2018) Malignant otitis externa: a retrospective analysis and treatment outcomes. Turk Arch Otorhinolaryngol 56(2):106–10
Mani N, Sudhoff H, Rajagopal S, Moffat D, Axon PR (2007) Cranial nerve involvement in malignant external otitis: implications for clinical outcome. Laryngoscope 117:907–910
Soudry E, Joshua BZ, Sulkes J, Nageris BI (2007) Characteristics and prognosis of malignant external otitis with facial paralysis. Arch Otolaryngol Head Neck Surg 133:1002–1004
Hern JD, Almeyda J, Thomas DM et al (1996) Malignant otitis externa in HIV and AIDS. J Laryngol Otol 110:770–775
Ress BD, Luntz M, Telischi FF et al (1997) Necrotizing external otitis in patients with AIDS. Laryngoscope 107:456–460
Berenholz L, Katzenell U, Harell M (2002) Evolving resistant pseudomonas to ciprofloxacin in malignant otitis externa. Laryngoscope 112(9):1619–1622
Chen YA, Chan KC, Chen CK, Wu CM (2011) Differential diagnosis and treatments of necrotizing otitis externa: a report of 19 cases. Auris Nasus Larynx 38(6):666–670
Gassab E, Krifa N, Sayah N, Khaireddine N, Koubaa J, Gassab A (2011) Necrotizing otitis externa: report of 36 cases. Tunis Med 89(2):151–156
Grandis JR, Branstetter BF, Yu VL (2004) The changing face of malignant (necrotizing) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis 4(1):34–39
Singh A, Al Khabori M, Hyder MJ (2005) Skull base osteomyelitis: diagnostic and therapeutic challenges in atypical presentation. Otolaryngol Head Neck Surg 133:121–125
Narozny W, Kuczkowski J, Stankiewicz C, Kot J, Mikaszewski B, Przewozny T (2006) Value of hyperbaric oxygen in bacterial and fungal malignant external otitis treatment. Eur Arch Otorhinolaryngol 263(7):680–684
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Kalaiarasi Raja and Sivaraman Ganesan: Conceptualized, Collected data,; Raghul Sekar: Wrote the article, Analysed data; Kalaiarasi Raja, Sunil Saxena, Arun Alexander: Revised article. All authors contributed to the study conception and design. The first draft of the manuscript was written by [Dr Raghul Sekar] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. Our Institute Research Ethics Committee has confirmed that no ethical approval was required.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sekar, R., Raja, K., Ganesan, S. et al. Clinical and Current Microbiological Profile with Changing Antibiotic Sensitivity in Malignant Otitis Externa. Indian J Otolaryngol Head Neck Surg 74 (Suppl 3), 4422–4427 (2022). https://doi.org/10.1007/s12070-021-03068-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-021-03068-9