Abstract
Osteoma is a slow growing, asymptomatic, benign bony tumor composed of compact and cancellous bones. Central, peripheral, and extra skeletal osteomas are the three types based on the site of origin. They are mostly observed on routine radiographic screening, mostly in the paranasal sinuses. Gnathic involvement is an uncommon occurrence, and if present, mandibular involvement is more frequently seen. Mostly, osteomas are small asymptomatic lesions and very rarely they become symptomatic and acquire larger size. Multiple osteomas are a feature of Gardner’s syndrome; however, solitary osteomas are non-syndromic. Oral health professional may be the first to diagnose Gardner’s syndrome as the osteomas may be initial manifestation of the disorder. Treatment protocol of osteomas varies based on the associated signs and symptoms. Small, asymptomatic cases are treated conservatively by periodic clinical and radiographic evaluation. However, larger, symptomatic lesions require surgical intervention. Herby, reporting an unusual case of Giant peripheral osteoma of the mandible. Our case is unique in few aspects because of its unusually large size (5 × 4 cm) and involvement of lingual aspect of the mandible in the region of sublingual fossa, with compression of the floor of mouth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An osteoma refers to a painless, gradually progressing benign bone neoplasm typified by proliferation of mature spongy and/or compact bone [1].
Osteomas can be delineated into three types based on the site of origin: Central, originates from the bony endosteum; peripheral, protrudes from the bone surface; or heterotopic, originates apart from bone in soft tissue (muscles) [2, 3]. Peripheral osteomas (Pos) exhibits centrifugal expansion from the periosteum, while central osteomas exhibit centripetal growth from the endosteum [4].
However, the recent World Health Organization (WHO) nomenclature delineates only two types-central and surface osteomas [2].
Osteomas are primarily confined to the craniofacial structures, with a site predilection for paranasal sinuses (primarily frontal sinus followed by ethmoid and maxillary sinus) [5]. Gnathic skeletal involvement is, however, rarely seen [6].
Generally, peripheral osteomas manifest as an asymptomatic slow growing bony mass, usually small in size [7]. On the contrary, large osteomas may result in facial disfigurement, and may displace and injure the adjoining framework [7, 8].
Individuals with multiple osteomas should be thoroughly appraised to rule out Gardner’s syndrome. As Gardner’s syndrome exhibit a genetic predisposition, new osteomas may appear after a few months or years. Hence, a periodic review every 4–6 months is essential for patients with Gardner’s syndrome [9,10,11].
Oral physicians should always bear in mind that most osteomas are an incidental finding on regular radiographic screening, hence, oral physicians should be familiar with the varied presenting manifestation of this clinical entity [12].
The treatment protocol relies on a myriad of factors-size, site, extent of the lesion along with the it’s proliferative pattern and accompanying manifestations. Asymptomatic osteomas are generally treated conservatively, and periodic imaging is recommended to observe the growth rate. However, surgical intervention is reserved for symptomatic cases [13, 14].
Here, we report a rare case of a giant solitary peripheral osteoma of mandible arising from the lingual aspect of mandibular body and occupying the floor of mouth. A brief review on the published literature of giant osteoma of mandible has also been presented.
Case Report
A 65-year-old female reported to the out-patient Department of Oral Medicine and Radiology, Saveetha Dental College and Hospitals, Chennai with a chief complaint of swelling beneath the right side of the tongue for the past 10 years. History reveals that the patient noticed an asymptomatic swelling beneath the right side of tongue 10 years back. The swelling showed slow progressive growth to attain the present size; however, there has been no increase in the size for the last 1–2 years. Negative history of facial trauma was elicited. Family history was unremarkable for gastrointestinal symptoms, and no similar swelling was noticed in any other part of the body. Dental history was positive for multiple tooth extractions.
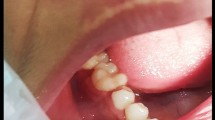
Physical examination was unremarkable with no constitutional symptoms. Extraoral examination revealed a firm, non-tender swelling in the right sub mental region with non-palpable regional lymph nodes (Fig. 1a). Intraoral examination revealed a solitary, pinkish-red, lobulated swelling of size 5 cm anteroposteriorly and 4 cm mesiodistally in the floor of the mouth on the right side. The swelling extended anteriorly to the symphysis region, posteriorly up to the distal aspect of the second molar, medially up to the base of tongue causing deviation of tongue postero-laterally, and laterally up to the lingual aspect of mandibular body and revealed a cranial growth. The swelling caused lateral and posterior displacement of tongue, thus, interfering with speech and mastication. The surface of swelling was smooth with no inflammatory component. The mouth opening was within the normal range with no alteration in lip, chin, and tongue sensations. The oral hygiene was poor with multiple missing teeth, generalized tooth mobility and cervical abrasion. On palpation, the inspectory findings such as size, site, shape, surface, and extent were confirmed. Palpatory findings suggested that the swelling was bony hard in consistency, non-tender, fixed to the mandible and does not yield on pressure. The borders of the swelling were well defined and merged with the base of the tongue and floor of the mouth (Fig. 1b, c).
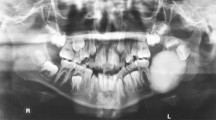
Mandibular occlusal radiograph and Lateral oblique radiograph revealed well-defined radio opacity in the right side of mandible, not surrounded by radiolucent rim (Fig. 2a, b) OPG showed a well delineated radio opaque lesion in right mandible. The radio-opacity extended antero-posteriorly from the right symphysis region to the distal aspect of second molar, and mesio-distally from the lingual aspect of right side of body of mandible to the floor of the mouth. The radio-opaque mass was not surrounded by a radiolucent rim. OPG also revealed multiple missing teeth, generalized interdental bone loss with ill-defined periapical pathology in relation to medially displaced second premolar (Fig. 2c).
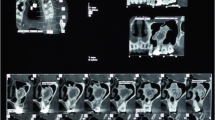
CT scan revealed a moderately sized pedunculated, hyper dense bone lesion of size 3.5 × 3.4x1.8 cm arising from the lingual aspect of body of mandible on the right side in the region of sublingual fossa. The lesion was seen compressing the adjacent floor of mouth (Fig. 3a, b).
Based on the history, clinical examination, and radiological features, provisional diagnosis of peripheral osteoma was considered, and Exostosis, odontoma, osteoid osteoma, osteoblastoma and ossifying fibroma were placed in the list of differential diagnosis.
Comprehensive blood parameters, liver and kidney function tests were in the normal range. The patient was instructed about the nature of the lesion and advised surgical intervention. After a written consent, mass was surgically resected in toto raising the mucoperiosteal flap. Healing was uneventful and no complications were noted (Fig. 4a). Histopathological examination of the decalcified tissue revealed mature compact lamellar bone trabeculae with minimal marrow spaces, and prominent osteoblastic activity, features consistent with a diagnosis of compact-type peripheral osteoma (Fig. 4b, c).
However, the patient was lost for further follow up.
Discussion
According to the recent WHO nomenclature, osteomas are classified as a benign neoplasm of well differentiated mature bone tissue [15], and Jaffe [16] first described the condition [15, 17].
A solitary peripheral osteoma of the gnathic skeleton is an uncommon occurrence, with a prevalence of less than 1%. However, mandibular involvement is more frequently seen, in contrast to the maxilla [6, 18].
Mandibular osteomas exhibit a site predilection for the angle and lower border of the body of mandible (mostly in association with the buccal cortical plate). Involvement of the lingual aspect of mandibular body, such as in our case, is an uncommon occurrence [6, 12, 19, 20].
Etiology of osteomas is still controversial. Some researchers have proposed the neoplastic or developmental basis of the entity [5, 9, 10, 18].
The embryological or developmental theory advocates development of osteomas from sutures between bones of diverse embryological origin. However, this theory could not be substantiated as osteomas usually progress in adults and not during childhood or adolescence [7, 20].
The persistent growth pattern exhibited by osteomas during adulthood is the most distinctive characteristic, thus, demarcating these lesions from other bony exostoses, and advocating the neoplastic theory of origin [10, 20]. However, other researchers deny the neoplastic origin because of the very slow growth rate.
Donohué Cornejo et al. [21] demonstrated the neoplastic activity by scintigraphy in an active giant osteoma of the mandible.
Furthermore, Bulut et al. [7], Kaplan et al. [10], Woldenberg et al. [22] proposed that osteomas may develop as a reactive lesion due to trauma, infection, or muscle activity.
Minor trauma may result in subperiosteal edema or bleeding and the muscle activity could locally elevate the periosteum, thus, triggering an osteoproliferative response that could be sustained by the unremitting muscle traction [10, 15, 20].
Most peripheral osteomas occur in proximity with muscle insertions (masseter, medial pterygoid, and temporalis); thus, supporting the reactive genesis secondary to muscle traction [6, 9, 10, 18, 22].
The inflammatory theory proposes that chronic infection of paranasal sinuses triggers proliferation of periosteum associated bone cells. However, it is still debatable to ascertain it is the infection or osteoma that develop earlier. Moreover, this theory fails to elucidate pathogenesis of osteomas in other sites [7].
The lesion in our patient was in contiguity to mandibular teeth with chronic periodontitis. The trigger factor for osteoma formation in our case could possibly be attributed to the poor oral hygiene leading to multiple tooth extractions.
Unilateral, well-circumscribed, sessile/pedunculated, mushroom shaped masses of size 1.5–4 cm and exhibiting a slow, persistent growth pattern generally summarizes the clinical features of peripheral osteomas [7, 20, 23, 24]. The term “gigantiform or huge osteoma” may be designated to large sized untreated osteomas (> 3 cm in size or 110gm in weight) [17, 20, 25].
Giant osteoma of the mandible is an exceptionally rare entity, and to the best of our knowledge (after thorough PUBMED, Scopus and Google scholar data base), only 30 cases of giant peripheral mandibular osteomas have been reported so far in the literature (Table 1).
Our case is unique in few aspects because of its unusually large size (5 × 4 cm) and involvement of lingual aspect of the mandible in the region of sublingual fossa, with compression of the floor of mouth.
Osteomas may be seen in any age group. However, most cases are seen in the 30–50-year age range, with a slight predilection to occur in males than in females [2, 3, 5, 8, 9, 12, 19, 26, 27].
However, other researchers documented that osteoma occur mostly in the younger age group with no gender predilection [7, 28, 29]. Our patient was 65-year-old female who noticed the swelling some 10 years back.
Generally, most POs are asymptomatic and are incidental finding on regular clinical and radiographic assessment. Gross facial asymmetry and swelling with hindered functions (difficulty in chewing, swallowing, breathing, and restricted mandibular movements) may be seen in larger lesions [7, 17, 19, 25, 28, 30]. Mandibular osteomas may also present alteration in bite and dentition [29].
The present case revealed a huge, solitary bony mass in the floor of mouth, and interfered with speech and mastication.
Various imaging techniques like occlusal radiographs, orthopantomogram (OPG), Waters projection, computerized tomography (CT), Cone beam computer tomography (CBCT), and magnetic resonance imaging (MRI) play an essential role in periodic evaluation of POs [15]. CT is an excellent imaging aid for diagnosis, anatomical location, and size determination of the lesion [8, 11, 17, 23, 28, 30].
Radiographically, POs appear as ovoid, well-defined, radiopaque, sessile/pedunculated lesions of size 1–4 cm in diameter, with a density comparable to that of normal bone [3, 4, 7, 9, 11, 18, 22, 25, 30]
These features are in coherence to our case report. These lesions usually do not damage the adjoining bony structures as reported in our case [3].
POs appear as well-circumscribed, lobulated, round-ovoid, sessile/pedunculated, mushroom shaped hyperdense lesions. Sessile osteomas are frequently connected to the bony cortex with a wide stalk, in contrast to the pedunculated lesions which have a narrow bony contact [7]. However, pedunculated lesions are not always apparent radiographically, hence, an incisional biopsy is imperative for a confirmatory diagnosis [28].
Microscopically, POs presents in 2 forms. Compact osteoma appears as solid lamellar bone mass with minimal marrow tissues; and Cancellous osteoma presents as trabeculae of mature lamellar bone trabeculae with interspersed fibrous or fatty marrow [2, 3, 9, 28, 31]. Generally, POs exhibit a pronounced osteoblastic activity. Most gnathic POs are microscopically compact osteomas, in coherence with the present case.
Exostoses, osteoid osteoma, osteoblastoma, peripheral ossifying fibroma, complex odontoma and late-stage central ossifying fibroma, must be contemplated in the differential diagnosis of POs.
Exostoses such as tori are common bony outgrowths (usually on the labial/buccal region of the alveolar bone), and are considered hamartomatous, developmental or reactive lesions, rather than true neoplasms. Varied growth pattern with growth cessation after puberty characterizes these lesions [3, 7, 19, 24, 26, 28].
Osteoblastoma and osteoid osteoma are rapidly growing, painful lesions chiefly affecting younger age group. Pain due to osteoblastoma decreases with aspirin, in contrast to osteoid osteoma, where pain diminution with aspirin does not occur [11, 19, 32].
Our patient reported an independent slow growth pattern and was symptom free during the entire course of the lesion, thus, ruling out the possibility of exostosis, osteoid osteoma and osteoblastoma.
Ossifying fibroma is a reactive lesion affecting the teenagers and young individuals. Reactive exuberant enlargement of the interdental papillae in maxillary anterior region, with a well-circumscribed, radiopaque mass not invading the bony cortex usually constitute the clinical and radiographic features [11, 24].
Central ossifying fibroma usually manifest as a well-defined radiopacity surrounded by a thin radiolucent line separating it from the adjoining bone. A sclerotic border may be present in the bone next to the lesion [19, 32]. Thin radiolucent line is the key distinguishing radiographic feature with POs [24].
Complex odontoma appears as a well delineated radiopaque mass encircled by a thin radiolucency, with a density greater than the abutting bone. [6, 19, 32].
Our case presented as a well-defined lobulated radiopaque mass without a radiolucent rim, thus ruling out the possibility of odontoma and ossifying fibroma.
Multiple osteomas in a patient may alert the physician for a possible Gardner’s syndrome. Multiple intestinal polyps (strong risk predisposition for malignant transformation), multiple skeletal osteomas/enostosis, and multiple impacted supernumerary teeth constitute the cardinal features of this syndrome. Osteomas usually precede the development of intestinal polyps, and oral physician may the first to diagnose this condition, thus, ameliorating the prognosis of the disorder [3, 5, 7, 8, 10].
Our patient did not report any gastrointestinal symptoms or dental anomaly. Family history and clinical examination was also inconclusive for Gardner’s Syndrome.
Varied treatment approach exists for POs. Smaller asymptomatic lesions generally require periodic radiographic evaluation, whereas, surgical excision is reserved for lesions which are large, disfiguring, symptomatic lesions exhibiting expeditious growth pattern and jeopardizing the functional ability [5, 7, 8, 12, 18, 19, 33].
The tumor mass was surgically resected in our patient, and healing occurred uneventfully. Recurrence of POs is an exceptionally uncommon occurrence. Only 3 cases of recurrence have been documented till date [34,35,36]. However, there has been no evidence of malignant transformation of POs [3, 5, 6, 11, 17, 18, 22, 28].
Conclusion
Osteomas are benign bone neoplasms exhibiting slow persistent growth pattern. Periodic clinical and radiographic assessment plays a key role in the management of osteomas. Oral health professional should be familiar with the presentation, as multiple osteomas are a feature of Gardner’s syndrome. CT is the imaging modality of choice. Recurrence after surgical intervention is extremely rare.
References
Fourcade A, Salmon B, Pelletier FL, Ejeil AL (2018) Peripheral osteoma of the mandibular crest: a short case study. J Oral Med Oral Surg 24:29–32
Kamimura R, Fukumoto C, Hasegawa T, Komiyama Y, Fujita A, Kawamata H (2020) A case of mandibular peripheral osteoma on the inferior border of the mandible. Oral Sci Int 00:1–5
Ragupathy K, Priyadharsini I, Sanjay P, Yuvraj V, Balaji TS (2015) Peripheral osteoma of the body of mandible: a case report. J Maxillofac Oral Surg 14(4):1004–1008
Ata-Ali J, Ata-Ali F (2019) Giant peripheral osteoma of the mandible simulating a parotid gland tumor. Braz J Otorhinolaryngol 85:393–395
Figueiredo NR, Meena M, Dinkar AD, Malik S (2014) Peripheral osteoma of the angle of mandible. Int J Oral Health Sci 4:33–36
Sayit AT, Kutlar G, Idilman IS, Gunbey PH, Celik A (2014) Peripheral osteoma of the mandible with radiologic and histopathologic findings. J Oral Maxillofac Radiol 2:35–37
Bulut E, Acikgoz A, Ozan B, Gunhan O (2010) Large peripheral osteoma of mandible. Int J Dent 834761. doi:https://doi.org/10.1155/2010/83476.
Saati S, Nikkerdar N, Golshah A (2011) Two huge maxillofacial osteoma cases evaluated by computed tomography. Iran J Radiol 8(4):253–257
Sayan NB, Uçok C, Karasu HA, Günhan O (2002) Peripheral osteoma of the oral and maxillofacial region: a study of 35 new cases. J Oral Maxillofac Surg 60(11):1299–1301
Kaplan I, Nicolaou Z, Hatuel D, Calderon S (2008) Solitary central osteoma of the jaws: a diagnostic dilemma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1106:22–29
Manjunatha BS, Das N, Sutariya R, Ahmed T (2013) Peripheral osteoma of the body of mandible. BMJ Case Rep 2013. doi:https://doi.org/10.1136/bcr-2013-009857.
Durãoa AR, Chilvarquerb I, Hayekb JE, Provenzanob M, Kendallc MR (2012) Osteoma of the zygomatic arch and mandible: report of two cases. Rev Port Estomatol Med Dent Cir Maxilofac 53(2):103–107
Olivares CM, Francisco PL, Claudio HM, Francisco PH (2020) Multiple mandibular osteomas not associated with gardner syndrome: case report and literature review. Res Rep Oral Maxillofac Surg 4(2):038. https://doi.org/10.23937/2643-3907/1710038
Viswanatha B (2012) Maxillary sinus osteoma: two cases and review of the literature. Acta Otorhinolaryngol Ital 32:202–205
Ozturk H, Torul D, Yuceer E, Karli R, Baris S (2018) Peripheral osteoma of mandibular angulus: analysis of the literature and report of a new case. Odovtos Int J Dental Sci 20(2):61–70
Jaffe HL (1935) Osteoid Osteoma: A benign osteoblastic tumor composed of osteoid and atypical bone. Arch Surg 31(5):709–728.
Sadeghi HM, Shamloo N, Taghavi N, Safi Y, Aghdashi F, Ismaeilnejad M (2015) Giant osteoma of mandible causing dyspnea: a rare case presentation and review of the literature. J Maxillofacial Oral Surg 14(3):836–840
Singhal P, Singhal A, Ram R, Gupta R (2012) Peripheral osteoma in a young patient: a marker for precancerous condition? J Indian Soc Pedod Prev Dent 30:74–77
Khandelwal P, Dhupar V, Akkara F (2016) Unusually large peripheral osteoma of the mandible—a rare case report. J Clin Diag Res 10(11):11–12
Kaya GŞ, Ömezli MM, Şipal S, Ertaş Ü (2010) Gigantic peripheral osteoma of the mandible: a case report. J Clin Exp Dent 2(3):160–162
Donohué-Cornejo A, Franco Garrocho LE, Albarrán-Vergara S, Gaitán Cepeda LA (2010) Active giant peripheral osteoma of the mandible. Presentation of one case with follow-up to 6 years. J Clin Exp Dent. 2(4):212–214
Woldenberg Y, Nash M, Bodner L (2005) Peripheral osteoma of the maxillofacial region. Diagnosis and management: a study of 14 cases. Med Oral Patol Oral Cir Bucal 10(2):139–142
Kerckhaert A, Wolvius E, van der Wal K, Oosterhuisa JW (2005) Giant osteoma of the mandible: case report. J Craniomaxillofac Surg 33(4):282–285
Soni S, Bhargav A (2014) Revisiting peripheral osteoma of the mandible with case series and review of literature Indian. J Otolaryngol Head Neck Surg 66(2):212–218
Ogbureke KU, Nashed MN, Ayoub AF (2007) Huge peripheral osteoma of the mandible: a case report and review of the literature. Pathol Res Pract 203:185–188
Sagar P, Jain K, Jain S, Bansal R (2017) Solitary giant osteoma of mandible. J Clin Diagn Res 11(12):04–06
Sachin Ram G, Ajila V, Hegde P, Babu S, Hegde S, Pillai DS et al (2019) Giant osteoma of the mandibular angle. Cukurova Med J 44(1):296–299
Gawande P, Deshmukh V, Garde JBJ (2015) Maxillofac Oral Surg 14(2):460–465
Almeida LE, De Oliveira Filho MA (2011) Giant mandibular condyle osteoma. J Craniofacial Surg 22:1147–1149
An SY, An CH, Choi KS (2006) Giant osteoma of the mandible causing breathing problem. Korean J Oral Maxillofac Radiol 36(4):217–220
Neville BW, Damm DD, Allen CM, Bouquot JE (2002) Oral & maxillofacial pathology, 2nd edn. W. B. Saunders Co, Philadelphia, p 567
White SC, Pharoah MJ (2004) Benign tumors of the jaws. In: White SC, Pharoah MJ (eds) Oral radiology: principles and interpretation, 5th edn, ch 21. Mosby, St. Louis, 410–458
Young-Kim D, Sung-Oh K (2015) A rare case of peripheral osteoma of the zygoma. Arch Plastic Surg 42(1):103–105
Bosshardt L, Gordon RC, Westerberg M, Morgan A (1971) Recurrent peripheral osteoma of mandible: report of a case. J Oral Surg 29:446–450
Shetty SK, Biddappa L (2015) Recurrence of a giant peripheral osteoma of mandible. J Maxillofac Oral Surg 14(1):452–456
Uenoyama A, Kodama Y, Tsurumaki H (2017) Two cases of peripheral osteoma arising in the mandible. Niigata Dent J 47(2):37–43
Acknowledgement
I would like to acknowledge the Department of Oral Medicine and Radiology, Faculty, and the patient at Saveetha Dental College & Hospitals, Chennai, Tamilnadu for their valuable support and contribution for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Human Participants and/or Animals
The patient and the attendants were informed about the nature of disease and treatment protocol.
Informed Consent
The patient was informed about the nature of disease and treatment protocol. Written informed consent was taken from patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasan, S. Giant Osteoma of the Mandible: Report of a Rare Case with Review of Literature. Indian J Otolaryngol Head Neck Surg 74 (Suppl 3), 4535–4542 (2022). https://doi.org/10.1007/s12070-021-02565-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-021-02565-1