Abstract
Primary ectopic meningiomas of the nasal cavity are rare tumours and thus, often not diagnosed and treated properly. In this case report we are going to discuss about our experience with a primary nasal meningioma involving bilateral nasal cavity with its diagnosis, management and histopathological features. A 28 year old female presented with nasal obstruction and nasal discharge for the past 1 year and swelling over right side of face for the past 8 months. Patient underwent surgical resection by a combined endoscopic and external approach under general anaesthesia. Histopathological evaluation confirmed the diagnosis of Transitional meningioma WHO grade 1. A repeat nasal endoscopy done one week after surgery, showed no evidence of any residual tumour. Due to ectopic meningiomas being relatively less common one must always exclude the presence of a central meningioma, which makes radiological investigations a must in such cases. As primary extra-cranial meningiomas have an unpredictable behaviour, this study should further aid in diagnosis and management of these tumours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are usually benign tumours of meningocytes or arachnoid cap cells of the arachnoid granulation tissue. They constitute around 15–20% of all intra-cranial tumours and are the most common non-glial intra-cranial neoplasm [1].
Among the 6–17% extra-cranial or extra-spinal meningiomas, head and neck is the most common site [1]. But the tumours seen are usually extension of the primary intracranial tumour. Primary ectopic tumours are only 2% of all meningiomas [1]. Primary ectopic meningiomas are defined as the ones that are not an extension of an underlying intracranial tumour nor they arise from foramina of any cranial nerve.
Meningioma of nose and paranasal sinuses constitutes only around 0.1% of all tumours of nose and paranasal sinuses [1]. The exact aetiology of these tumours is still unknown. In this report we are going to discuss about a rare case of extra-cranial meningioma operated in our department.
Case Report
A 28 year old female presented with nasal obstruction and nasal discharge for the past one year and swelling over right side of face for the past 8 months.
To start with patient had nasal obstruction and rhinorrhoea one year back. The nasal obstruction was initially only on the right side and minimal, so didn’t bother the patient but gradually became bilateral and was associated with nasal discharge which was foul smelling, mucopurulent and aggravated during episodes of upper respiratory infections. Patient also had history of decreased sense of smell perception.
There was history of occasional headache for the past 1 year, severe in intensity, pricking in nature, frontal in location and aggravated on bending forward and partially relieved on taking oral analgesics.
She noticed fullness on right side face 8 months back that gradually developed into a swelling which insidious in onset, painless, gradually enlarging to reach the current size.
There was no history of any blood tinged discharge, no history suggestive of allergy or any post nasal drip. There is was no history of any visual disturbances. Patient does not give history of trauma or any previous surgical intervention.
On examination there was presence of an irregular swelling of size 4 × 3 cm on right side of face and dorsum of nose causing complete obliteration of the tear trough and nasojugal groove. Skin over the swelling was normal in appearance. Swelling was soft to firm in consistency, non-tender and fixed to underlying structures.
On anterior rhinoscopy, there was presence of thick, purulent, foul smelling discharge in bilateral nasal cavity. There was presence of pinkish polypoidal mass completely obliterating the right nasal cavity (Fig. 1). Posterior rhinoscopy was normal.
Examination of the ear, oral cavity and neck revealed no abnormalities. Ocular examination revealed normal vision and no restriction in ocular movements. There was no proptosis or any displacement of the eye. Neurological examination was within normal limits and patient showed no signs of neurofibromatosis.
A diagnostic nasal endoscopy was done that revealed a presence of a polyp-like mass in bilateral nasal cavity, more on the right side. It seemed to be arising from roof of the nasal cavity and septum. Mucosa over the mass was intact. The mass didn’t shrink on application of decongestants. On probing, the probe couldn’t be passed medially and superiorly. The mass was sensitive but did not bleed on touch. Bilateral middle turbinate were pushed laterally.
Based on these findings, radiological evaluation was deemed necessary. Preoperative imaging including MRI and CECT was done to rule out any intracranial extension and to see the extent of the tumour.
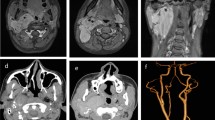
MRI of face and brain revealed presence of an ill-defined mixed signal intensity lesion (T1-Hypointense, T2- heterogeneously hyperintense, STIR- hyperintense) of 3.4 × 3 × 2.3 cm size in bilateral nasal cavity with suspicious origin from cribriform plate of ethmoid. Antero-laterally on right side there was erosion of right lateral nasal cartilage with superficial component measuring 1.3 × 2.8 × 3.3 cm in region of right nasal ala and superior extension till medial canthus of right eye. There was block of bilateral maxillary ostia leading to mucosal thickening in bilateral maxillary, frontal and ethmoidal sinuses. There was no obvious intracranial extension.
Contrast enhanced CT of paranasal sinuses and nose revealed similar findings with maintained cortical regularity of bilateral lamina papyracea with no obvious intra-orbital extension. There was also ill defined mixed lytic and sclerotic bony hyperostosis involving the nasal septum, perpendicular plate of ethmoid, bilateral nasal bones and medial wall of maxillary sinus (Fig. 2).
NCCT PNS and nose axial cuts showing. a ILL defined heterogeneously enhancing lesion noted occluding bilateral ethmoid air cells and bilateral superior and middle meatus of nasal cavity measuring ~ 4.2 × 3.5x3.3 cm (APxTRAxCC). b An exophytic irregular enhancing component along the right nasal ala in the subcutaneous plane measuring ~ 3.1 × 1.3 cm
The radiological investigations were also inconclusive and patient was taken up for an endoscope guided biopsy and fine needle aspiration cytology of the external component.
On histopathological evaluation there was moderate to high cellularity with cells arranged in cohesive clusters, syncytial sheets, whorls and nodules (Fig. 3). Individual cells had round to oval nuclei with stippled to vesicular chromatin and moderate fragile cytoplasm. There was increased vascularity with capillaries in between and the surrounding clusters. Based on these findings a diagnosis of meningioma was made.
Surgical resection was undertaken by a combined endoscopic and external approach under general anaesthesia with written informed consent. The endoscopic approach was facilitated with the help of a posterior septectomy and externally lateral rhinotomy was performed.
First the tumour was debulked and removed piecemeal with the help of a microdebrider under endoscopic guidance. The external component of the mass was removed in toto via lateral rhinotomy.
Intraoperatively the mass was mainly present in the right nasal cavity firmly adherent to the underlying nasal bones. The mass was rubbery, friable and didn’t bleed much on removal. There was no bony erosion of orbit or any intra-cranial extension. The external component of the mass was seen to be arising from the junction between nasal bone and frontal process of the maxilla. The underlying hyperostotic part of nasal bone was chiselled out. The olfactory mucosa was preserved but the nasal mucosa lining the nasal bones and involved by the tumour, was removed. Similarly, the mass on the left side was also removed piecemeal and had similar characteristics. Bilateral nasal cavity was packed.
Both the external and internal component of the mass were sent for histopathological evaluation separately. Histopathological evaluation showed presence of cells in whorls and syncytial nodules with dispersed chromatin, conspicuous nucleoli, nuclear inclusions, inconspicuous mitotic activity and psammoma bodies that confirmed the diagnosis of Transitional meningioma WHO grade 1 (Fig. 4).
The post-operative period was uneventful with no evidence of any CSF leak or any periorbital oedema. Anterior nasal pack was removed on second post-operative day.
A repeat nasal endoscopy done one week after surgery, showed no evidence of any residual tumour (Fig. 5). There was also significant improvement in the nasal ventilation and olfactory function of the patient postoperatively.
Patient has been kept on regular follow up every month till date with no evidence of recurrence.
Discussion
Farr et al. reviewed 405 meningiomas and found that the orbital cavity was the most common location of extra-cranial meningiomas associated with an intracranial mass [2]. They can also occur in the ear, parotid, neck, pharynx, mandible, and skin [3].
Our present patient was diagnosed with primary ectopic meningioma of the nasal cavity. This means it does not have any connection to intra-cranial structures. This diagnosis was made by using classification given by Hoye et al. [4] which encompasses the major aetiologies.
The pathogenesis of these extra-cranial ectopic meningiomas is described as a fusion line inclusion that is trapping of the meningiocytes during the closure of midline structures. In cases of meningiomas that are not near the cranial nerves or fusion lines, the origin is attributed to metaplasia of undifferentiated mesenchymal cells.
A study with 146 cases of extra-cranial head and neck meningiomas, showed 59 were in skin and scalp, 26 in middle ear, 17 in nasal cavity, 2 in temporal bone and one in the parotid gland. They also concluded that meingiomas have slow growth, good prognosis and longest survival in younger patients and complete resection [5].
Anosmia is usually the first symptom but they can also present with headache, nasal obstruction and visual disturbances [6].
Diagnosis of these tumours is challenging due to their rarity and presentation similar to nasal polyposis. It is usually made with the help of CT scan or MRI to check for any intracranial component or any involvement of skull base. Final diagnosis is made by biopsy as FNAC can be at times inconclusive. Hyperostosis of the surrounding is one classical finding in cases of meningioma that was also present in this case.
Based on histopathology, WHO has classified Meningiomas into three grades. Our case was Grade I which means it is typical or benign.
Histologically, meningiomas can also be divided into different histological subtypes according to the predominant cellular morphology. Our case was transitional which implies mixed cellular morphology.
The majority of extra-cranial meningiomas of the paranasal sinuses have been syncytial. The transitional type as in this case consists of whorls of spindle cells surrounded by fibrous tissue. These whorls may have central vessels or hyalinized or calcified collagen producing psammoma bodies [1].
Although meningiomas are usually benign they show predilection towards invading foramina and can also cause pressure necrosis thus involving more than one cavity. In this case, non-involvement of any cranial nerves or orbit proves its benign nature.
Zulch stated more than the histopathological grading a complete extirpation of the tumour is more important to prevent recurrence [7].
There are various methods of treatment that are being considered for meningiomas including surgical, radiotherapy, chemotherapy, proton irradiation and stereotactic radiosurgery.
Out of the above the mainstay of treatment is surgical that includes a radical resection. This includes removal of the tumour, removal of intracranial connection if any along with the hyperostotic bone. Radical resection offers the best chance in preventing any recurrence (72% at 5 years) [8].
Chances of recurrence is higher in cases with incomplete surgical excision, higher histological grades, nucleolar prominence, more than 2 mitosis per 10 high power field, heterogenous tumour contrast enhancement on CT scan and paediatric age group.
In certain cases like unresectable malignant tumours, recurrent cases and patients in which surgery cannot be done chemotherapy and radiotherapy are considered the primary treatment.
According to Rushing et al., 96% of extra-cranial meningiomas were somatostatin receptor positive, 89% EGF receptor positive, 76% progesterone receptor positive and 19% oestrogen receptor positive [5]. Thus, the role of Tamoxifen and RU-486, an anti-progesterone as a potential treatment are investigated [6].
Conclusion
In this case due to inconclusive clinical and radiological evaluation various differentials were kept including frontoethmoidal mucocele, meningioma, olfactory neuroblastoma, neurofibroma and malignancy of nose and paranasal sinuses. The final diagnosis could only be made on the basis of histopathological evaluation and due to rarity of this tumour meningioma was not kept as one of the first differentials.
Due to ectopic meningiomas being relatively less common one must always exclude the presence of a central meningioma, which makes radiological investigations a must in such cases.
As primary extra-cranial meningiomas have an unpredictable behaviour, this study should further aid in diagnosis and management of these tumours.
References
Aiyer RG, Prashanth V, Ambani K, Bhat VS, Soni GB (2013) Primary extracranial meningioma of paranasal sinuses. Indian J Otolaryngol Head Neck Surg 65:384–387
Farr HW, Gray GF Jr, Vrana M, Panio M (1973) Extracranial meningioma. J SurgOncol 5:411–420
Landini G, Kitano M (1992) Meningioma of the mandible. Cancer 69:2917–2920
Hoye SJ, Hoar CS, Murray JE (1960) Extracranial meningioma presenting as a tumor of the neck. Am J Surg 100:486–489
Rushing EJ, Bouffard JP, McCall S, Olsen C, Mena H, Sandberg GD et al (2009) Primary extracranialmeningiomas: an analysis of 146 cases. Head Neck Pathol 3:116–130
Zhang Y, Teng WQ, Chen XP, Wu J (2015) Ectopic meningioma in the bilateral nasal olfactory cleft: a case report and literature review. OncolLett 9:1743–1746
Zülch KJ (1979) Histological typing of tumors of the central nervous system. Geneva: World Health Organization. http://books.google.com/books?id=x5prAAAAMAAJ
Sharma J, Pippal S, Sethi Y (2006) A rare case of primary nasoethmoidal meningioma. Indian J Otolaryngol Head Neck Surg 58:101–103
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rai, T., Shenoy, V.S., Varshney, P. et al. Primary Ectopic Meningioma of Nasal Cavity: A Rare Presentation. Indian J Otolaryngol Head Neck Surg 74 (Suppl 2), 1197–1201 (2022). https://doi.org/10.1007/s12070-020-02285-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-020-02285-y