Abstract
We investigated the effectiveness of N-acetyl cysteine (NAC) and curcumin, which have known antioxidant and anti-inflammatory effects, in reducing acoustic trauma. We randomly divided 40 adult male rats into four groups: a control group (group 1), a curcumin group (group 2), a NAC group (group 3), and an ethyl alcohol group (group 4). The rats were exposed to 110 dB sound at a frequency of 4 kHz for 2 h to simulate acoustic trauma. Group 1, group 2, group 3, and group 4 received 1 ml saline, 200 mg/kg curcumin, 350 mg/kg NAC, or 1 ml ethyl alcohol, respectively, intraperitoneally 30 min before and 24 and 48 h after acoustic trauma. Distortion product otoacoustic emissions (DPOAEs) were recorded before and after the acoustic trauma, and 72 h after drug administration. In group 2, signal-to-noise ratio (SNR) values in frequencies of 1000 Hz, 1500 Hz, and 4000 Hz decreased in the second measurements when compared to the first, and showed improvements in the third measurements in comparison to the second ones. In group 3, SNR values decreased in the second measurements, but only the values at 6000 Hz were found to be statistically significant (p = 0.007). The values in the third measurements were statistically significant when compared to the second ones. There was a statistically significant difference in the third measurements in both groups 2 and 3, possibly due to curcumin and NAC treatment. This study showed that curcumin and NAC may be effective against noise-induced hearing loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustic trauma occurs as a result of damage to hearing mechanisms in the inner ear after exposure to excessive noise (> 85 dB). Acoustic trauma is the most important cause of noise-related hearing loss in adults [1].
Exposure to high-intensity noise (130 dB SPL) causes direct mechanical damage in hairy cells and support cells in the organ of Corti. As a result of this damage, the biochemical complex cascade is activated in hairy cells and reactive oxygen species (ROS) are formed. Ischemia, exotoxic damage, metabolic fatigue, and ionic imbalance occur in the inner ear fluids [2]. After the development of trauma-induced hypoxia, ROS cannot be removed due to impaired blood circulation and accumulate in tissues. Accumulation of radicals leads to progressive cell damage, and sensorineural hearing loss develops [3, 4]. Studies on acoustic trauma have emphasized that cochlear damage develops due to this ROS increase and hypoxia. To reduce or prevent this damage, treatment has focused on antioxidant agents [1].
Curcumin is a natural product of the curcumin longa plant. It has been reported to have antioxidant, anti-inflammatory, immunomodulatory, antitumoral, and anti-psoriatic effects [5]. Curcumin is believed to mediate these effects via limiting production of the proinflammatory cytokines, IL-β and IL-18, and activation of caspase-1 [6].
NAC is the N-acidified derivative of L-cysteine, a natural amino acid. An antioxidant that contains thiol and can pass through the cell membrane, NAC plays a role in the formation of cysteine and is involved in glutathione synthesis. The resulting glutathione protects the cochlear mitochondria against ROS and reduces oxidative stress in the cochlea [3, 7].
In this study, ethyl alcohol was used to dissolve curcumin. Therefore, ethyl alcohol group was created to eliminate the possible effects of ethyl alcohol.
In clinical studies on acoustic trauma, excessive acoustic stimulation has been shown to reduce the amplitude of otoacoustic emissions. In these and similar studies, DPOAEs and auditory brainstem response (ABR) tests have been used to detect acoustic trauma [8].
Our study sought to investigate whether the use of curcumin and NAC has positive effects on the prevention and reduction of hearing loss due to acoustic trauma.
Methods
Subjects and Anesthesia Procedure
The research was carried out with the approval of Karadeniz Technical University Animal Experiments Local Ethics Committee with the protocol number 2014/21. Adult Sprague Dawley rats (n = 40) weighing 250–300 g were used. Otoscopic examination observed that the external ear canal and tympanic membrane of all subjects were normal. Subjects were randomly divided into four groups: The control group (group 1), curcumin-acoustic trauma group (group 2), NAC-acoustic trauma group (group 3), and ethyl alcohol-acoustic trauma group (group 4).
Anesthesia was provided with 50 mg/kg intraperitoneal (i.p.) ketamine hydrochloride (Ketalar®, Parke-Davis Eczacibasi, Istanbul, Turkey) and 10 mg/kg i.p. xylazine hydrochloride (Alfazyne®, Alfasan International B.V. Woerden, The Netherlands).
Test Procedure
All measurements were carried out by the same person in the audiology unit silent cabin. Anesthesia was applied to all subjects during the procedure. Acoustic trauma was created by exposing the subjects to audio noise via an audiometry device (Interacoustics AC 40) at a frequency of 110 dB SPL for 2 h at a frequency of 4 kHz. Dosing of the study groups was as follows:
Group 1 (Control group, n = 10): 1 ml of saline was applied i.p. to subjects 30 min before and 24 h and 48 h after acoustic trauma.
Group 2 (curcumin-acoustic trauma group, n = 10): 200 mg/kg of Curcuma longa (Turmeric) (Sigma/catalog no: C1386) dissolved in ethyl alcohol and prepared as a 1 ml solution was applied i.p. to subjects 30 min before and 24 h and 48 h after acoustic trauma.
Group 3 (NAC- acoustic trauma group, n = 10): 350 mg/kg NAC (Husnu Arsan Ilaclari, Istanbul, Turkey) as a string in 1 ml saline solution was applied i.p. to subjects 30 min before and 24 h and 48 h after acoustic trauma.
Group 4 (ethyl alcohol-acoustic trauma group, n = 10): 1 ml of ethyl alcohol was applied i.p. to the subject 30 min before and 24 h and 48 h after acoustic trauma.
Otoacoustic Emissions and Recording
All measurements were made by the same person in a soundproofed and constant-temperature quiet cabin. DPOAE was measured in both ears with the IHS-EP SMART OAE-ABR combined device. Responses were recorded by the suitable probe for the external ear canal of the subjects. The intensity of the frequencies inside the stimulus given during DPOAE measurements was taken as L1 (65 dB SPL) for frequency f1 and L2 (55 dB SPL) for frequency f2. Signal-to-noise ratios (SNR) were obtained for each mentioned frequency by measuring with stimulus. The ratio between the frequencies f2 and f1 (f2 / f1) was kept at 1.22. Target stimulation was determined as 80 dB and the DPOAE test was applied.
The responses were measured with the microphone in the external ear canal at a frequency of 2f1-f2. Measurements were recorded at the geometric mean of f1 and f2 at frequencies 1003, 1409, 2000, 3991, 5649 Hz. In these measurements, the noise rejects ratio was kept at a maximum of 10 dB.
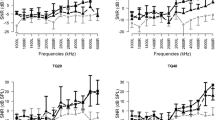
DPOAE recordings were obtained before acoustic trauma (1st measurement), after acoustic trauma (2nd measurement), and 72 h after drug administration (3rd measurement). SNR values obtained during DPOAE of all groups were compared between groups and within groups.
Statistical Analysis
All data were analyzed using the SPSS 13.0 statistics program. The Wilcoxon Signed-rank Test was used for comparisons within the group; the Kruskal–Wallis Test was used for comparison between the groups. The Mann–Whitney U test was used in posthoc analysis between groups, and the Bonferroni correction was applied. Right and left ear measurement results were evaluated with the Paired Samples Test. A p-value < 0.05 was considered significant.
Results
Baseline values were calculated for all rats using the DPOAE test before the acoustic trauma (1st measurement). After the acoustic trauma, DPOAE was performed again (2nd measurement). Finally, DPOAE measurements were made after drug administration at 72 h (3rd measurement).
No statistically significant difference was found between the right and left ears in all three measurements in all four groups (p > 0.05). For this reason, the averages of the variables obtained for the right and left ears were taken and these mean values were used in comparisons.
Intra-Group Comparisons
In group 1, a statistically significant decrease in the SNR values was observed between the 1st measurement and the 2nd measurement (p < 0.05). The decrease in SNR values between the 1st measurement and the 3rd measurement was significant (p < 0.05) at all frequencies except 1000 Hz. There was no statistically significant difference in SNR values between the 2nd and 3rd measurements in this group (p > 0.05).
In group 2, there was a decrease in the values in all frequencies between the 1st measurement and the 2nd measurement; however, only the decreases at 1000, 1500, and 4000 Hz were statistically significant. Between the 2nd and 3rd measurements a statistically significant (p < 0.05) improvement in SNR values was detected at all frequencies. There was no statistically significant difference in SNR values between the 1st and 3rd measurements (p > 0.05).
In group 3, there was a decrease in SNR values between the 1st and 2nd measurements, though this decrease was found to be significant only at 6000 Hz. A statistically significant improvement in SNR values was observed between the 2nd measurement and the 3rd measurement at all frequencies. There was no statistically significant difference in SNR values between the 1st measurement and the 3rd measurement.
In group 4, a statistically significant decrease in SNR values at the 2000, 4000, and 6000 Hz frequencies was detected between the 1st and 2nd measurements. There was no statistically significant difference in SNR values between the 2nd measurement and the 3rd measurement.
Inter-Group Comparisons
The SNR measurements before and after acoustic trauma (1st measurement and 2nd measurement) did not show a statistically significant difference between the four groups (p > 0.05).
When the SNR values obtained in the third measurements were compared, statistically significant differences were found at 1500, 2000, 4000, and 6000 Hz. For these frequencies, at which there was determined to be difference between the groups, a posthoc comparison between the groups was performed (p < 0.008). (Table 1).
We found statistically significant differences between the 1st group and the 2nd group at 4000 Hz, between the 1st and the 3rd group at 4000 and 6000 Hz, between the 2nd and 4th groups at 4000 Hz, and between the 3rd and the 4th group at 4000 and 6000 Hz. (Table 2).
Discussion
Oxidative stress plays a role in the pathology of trauma-related hearing loss. Oxidative stress caused by trauma leads to mitochondrial injury, activation of the cell death pathway, activation of inflammatory mediators, and elevated lipid peroxidase level. Due to ROS formation, permanent hearing loss occurs as a result of the activation of this cascade. Antioxidant treatments can play a role in blocking this cascade [2].
In the present study, the efficacy of curcumin and NAC, known to be effective in preventing hypoxia and ROS, has been evaluated via measuring the damage caused by acoustic trauma in the cochlea.
ABR and DPOAE tests can be used to demonstrate the effects of acoustic trauma. After acoustic trauma, outer hair cells are affected first in the cochlea. DPOAE, measured from the external ear canal and evaluating the function of the outer hair cells, is a non-invasive, short-term test that can be used in acoustic trauma [8]. DPOAE was performed before and after the sound exposure in all groups in this study; the decrease in DPOAE measurements after the exposure showed that acoustic trauma occurred.
Many studies have investigated the properties of curcumin, the first of the antioxidants used in the study, particularly noting its antioxidant, antifibrotic, anti-inflammatory, anticancer, and apoptotic effects. Curcumin has been shown to reduce the production of proinflammatory cytokines such as IL-1, IL-2, IL-6, IL-8, TNF-A, IL-12, and to inhibit NFkB, AP-1, JAK-kinase, cyclooxygenase, and lipoxygenase [9].
Due to the possibilities suggested by these effects, this study investigated whether curcumin would be effective in preventing damage to the cochlea due to acoustic trauma. Salehi et al. investigated the efficacy of dexamethasone and curcumin in reducing cisplatin ototoxicity, and showed that this combination reduces toxic damage in auditory cells and has a protective effect on hearing loss [10]. Fetoni et al. also showed curcumin reduces hearing loss and extends the life of outer hair cells in cisplatin ototoxicity [11]. Yamaguchi et al. showed that curcumin and its preparations have a protective effect in hearing loss due to repeated noise exposure [12].
In a study evaluating the effects of EF-24 (an analogue of curcumin) and cisplatin on the auditory and vestibular system, the combined use of EF-24 and cisplatin was reported to prevent the damage caused by cisplatin treatment in the inner ear [13]. Curcumin has also been reported to have a role in preventing fibroblast damage in cochlear support tissues as a result of noise exposure [14]. Soyalıç et al. used DPOAEs measurements and histopathological and immunohistochemical methods to show that curcumin is protective against acoustic trauma in cochlear tissues [15]. Similar to these studies, our study found a statistically significant improvement in SNR values between the 2nd and 3rd measurements in group 2 (curcumin-acoustic trauma group), while SNR values between the 3rd measurement and the 1st measurement were found to be similar. These results show the potential for curcumin to have positive effects on the recovery from acoustic trauma.
The effects of NAC on oxidative stress have been studied both in-vivo and in-vitro models. NAC plays a role in free radical elimination, GSH production, and prevention of mitochondrial damage. It also acts as a glutamate exotoxin inhibitor, lipid peroxidase inhibitor, and necrosis inhibitor. These protective effects have been investigated at different drug dosages and in acoustic trauma models [16]. Ada et al. showed that NAC reduces cochlear damage resulting from acoustic trauma in rats [17]. Another study used the auditory brainstem response (ABR) test to evaluate acoustic trauma in rabbits, and found that NAC provided a protective effect in the formation of temporary and permanent hearing loss [1]. Rosenhall et al. showed that the use of NAC after acoustic trauma reduces the risk of transient hearing loss in soldiers [18]. In our study, SNR values decreased in group 3 (NAC-acoustic trauma group) in the after-trauma measurement (2nd measurement), though this decrease was not statistically significant. This suggests that NAC has a protective effect during trauma. Also, the third measurement showed a statistically significant improvement in SNR values compared to the second measurement, implying that NAC is effective in improving hearing after acoustic trauma. Thus, our study observes that the use of NAC has a protective effect during acoustic trauma and contributes positively to post-traumatic healing.
References
Motalebi Kashani M, Saberi H, Hannani M (2013) Prevention of acoustic trauma-ınduced hearing loss by N-acetylcysteine administration in rabbits. Arch Trauma Res. 1:145–150. https://doi.org/10.5812/atr.7839
Ewert DL, Lu J, Li W, Du X, Floyd R, Kopke R (2012) Antioxidant treatment reduces blast-induced cochlear damage and hearing loss. Hear Res. 285:29–39. https://doi.org/10.1016/j.heares.2012.01.013
Choi CH, Chen K, Vasquez-Weldon A, Jackson RL, Floyd RA, Kopke RD (2008) Effectiveness of 4-hydroxy phenyl N-tert-butylnitrone (4-OHPBN) alone and in combination with other antioxidant drugs in the treatment of acute acoustic trauma in chinchilla. Free Radic Biol Med 44:1772–1784. https://doi.org/10.1016/j.freeradbiomed.2008.02.005
Colombari GC, Rossato M, Feres O, Hyppolito MA (2011) Effects of hyperbaric oxygen treatment on auditory hair cells after acute noise damage. Eur Arch Otorhinolaryngol 268:49–56. https://doi.org/10.1007/s00405-010-1338-4
Miquel J, Bernd A, Sempere JM, Díaz-Alperi J, Ramírez A (2002) The curcuma antioxidants: pharmacological effects and prospects for future clinical use. A review. Arch Gerontol Geriatr 34:37–46. https://doi.org/10.1016/s0167-4943(01)00194-7
Thiyagarajan M, Sharma SS (2004) Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci 74:969–985. https://doi.org/10.1016/j.lfs.2003.06.042
Pisani A, Riccio E, Andreucci M et al (2013) Role of reactive oxygen species in pathogenesis of radiocontrast-induced nephropathy. Biomed Res Int. 2013:868321. https://doi.org/10.1155/2013/868321
Nottet JB, Moulin A, Brossard N, Suc B, Job A (2006) Otoacoustic emissions and persistent tinnitus after acute acoustic trauma. Laryngoscope 116:970–975. https://doi.org/10.1097/01.MLG.0000216823.77995.13
Maheshwari RK, Singh AK, Gaddipati J, Srimal RC (2006) Multiple biological activities of curcumin: A short review. Life Sci 78:2081–2087. https://doi.org/10.1016/j.lfs.2005.12.007
Salehi P, Akinpelu OV, Waissbluth S et al (2014) Attenuation of sisplatin ototoxicity by otoprotective effects of nanoencapsulated curcumin and dexamethasone in a guinea pig model. Otol Neurotol. 35:1131–1139. https://doi.org/10.1097/MAO.0000000000000403
Fetoni AR, Eramo SL, Paciello F et al (2014) Curcuma longa (curcumin) decreases in vivo cisplatin-induced ototoxicity through heme oxygenase-1 induction. Otol Neurotol. 35:169–177. https://doi.org/10.1097/MAO.0000000000000302
Yamaguchi T, Yoneyama M, Onaka Y, Imaizumi A, Ogita K (2017) Preventive effect of curcumin and its highly bioavailable preparation on hearing loss induced by single or repeated exposure to noise: A comparative and mechanistic study. J Pharmacol Sci 134:225–233. https://doi.org/10.1016/j.jphs.2017.07.003
Monroe JD, Millay MH, Patty BG, Smith ME (2018) The curcuminoid, EF-24, reduces cisplatin-mediated reactive oxygen species in zebrafish inner ear auditory and vestibular tissues. J Clin Neurosci. 57:152–156. https://doi.org/10.1016/j.jocn.2018.09.002
Haryuna TS, Riawan W, Nasution A, Ma’at S, Harahap J, Adriztina I (2016) Curcumin reduces the noise-exposed cochlear fibroblasts apoptosis. Int Arch Otorhinolaryngol. 20:370–376. https://doi.org/10.1055/s-0036-1579742
Soyalıç H, Gevrek F, Karaman S (2017) Curcumin protects against acoustic trauma in the rat cochlea. Int J Pediatr Otorhinolaryngol. 99:100–106. https://doi.org/10.1016/j.ijporl.2017.05.029
Fetoni AR, Ralli M, Sergi B, Parrilla C, Troiani D, Paludetti G (2009) Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital 29:70–75
Ada S, Hanci D, Ulusoy S et al (2017) Potential protective effect of N-acetyl cysteine in acoustic trauma: An experimental study using scanning electron microscopy. Adv Clin Exp Med. 26:893–897. https://doi.org/10.17219/acem/64332
Rosenhall U, Skoog B, Muhr P (2019) Treatment of military acoustic accidents with N-Acetyl-L-cysteine (NAC). Int J Audiol 58:151–157. https://doi.org/10.1080/14992027.2018.1543961
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
İskender Emekli, Z., Şentürk, F. & Bahadir, O. Protective Effects of Curcumin and N-Acetyl Cysteine Against Noise-Induced Sensorineural Hearing Loss: An Experimental Study. Indian J Otolaryngol Head Neck Surg 74 (Suppl 1), 467–471 (2022). https://doi.org/10.1007/s12070-020-02269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-020-02269-y