Abstract
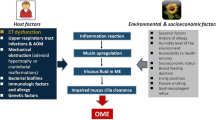
Eosinophilic otitis media (EOM) is an inflammatory chronic disease of the middle ear, characterized by the presence of a particularly viscous effusion with a high content of protein toxins of eosinophilic origin in the middle ear cavity. The pathology has relationship with bronchial asthma, allergic rhinitis and chronic rhinosinusitis with nasal polyps. EOM is characterized by a sluggish course, a tendency to relapse, which can lead to a gradual hearing decrease up to complete deafness. In this paper, we reviewed the international literature with special attention to pathogenesis and treatment management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Eosinophilic otitis media (EOM) is an inflammatory chronic disease of the middle ear, characterized by the presence of a particularly viscous effusion with a high content of protein toxins of eosinophilic origin in the middle ear cavity, as well as a severe course with a progression to hearing loss up to deafness.
Koch presented this pathology in 1947 for the first time in the scientific literature. He described cases of chronic otitis media with very viscous eosinophilic enriched secretions, accompanied by swelling of the middle ear mucous with a background of allergic rhinitis [1].
The term “eosinophilic otitis media” was first proposed in 1994 by Tomioka et al., who described three clinical cases of sluggish otitis media with a large number of eosinophils in the effusion, regardless of type I hypersensitivity reactions. They also indicated the relationship of this pathology with bronchial asthma (BA), allergic rhinitis (AR) and chronic rhinosinusitis with nasal polyps (CRSwNP) [2].
Epidemiology
The disease is most extensively learnt in Japan, which, probably, can be associated with a higher prevalence of nosology in this region. Thus, according to the results of a questionnaire survey of 1409 ENT—clinics throughout the country, about 340 patients with EOM were identified [3]. The disease developed mainly in women aged 50 to 60 years, both ears were affected in 81% of patients. Concomitant pathology of the nasal cavity and paranasal sinuses were detected in 74% of cases: 75% of patients had a history of chronic rhinosinusitis or CRSwNP in combination with BA that arise already in adulthood. Sensorineural hearing loss developed in 47% of patients, and deafness was observed in 6% of clinical cases [3]. In the majority of EOM patients identified in this survey, the first episodes of the disease appeared more than 10 years ago. In publications from Europe and USA, we have found only single descriptions of the clinical cases of EOM [4,5,6,7,8].
Pathogenesis of EOM
According to the literature, EOM mostly develops in adulthood on the background of rhinosinusitis and BA. The pathological process in the middle ear in most cases breaks out approximately 10 years after the development of lung pathology. Thus, EOM with the similar to BA pathogenesis mechanisms, can be considered as the endpoint of the chronic eosinophilic inflammatory process of the respiratory tract. [9]. When interacting with antigens, respiratory tract epithelial cells release inflammatory mediators (TSLP, IL-33, IL-25, etc.), which in turn stimulate the production of cytokines (IL-4, IL-5, IL-13, etc.) T2-helpers, starting an inflammatory process similar to that in BA [10, 11]. An increase in IL-5 expression induces the differentiation of the eosinophilic pool in the bone marrow and stimulates the migration of eosinophils to the bloodstream. Chemokines, for example, eotoxin, are produced by epithelial cells and fibroblasts and further facilitate the migration of eosinophils into the mucous membrane of the respiratory tract. After that the eosinophils are accumulated in the middle ear cavity as a result of the directed migration [12, 13].
Eustachian tube disfunction and inflammation of the nasal mucosa are important factors in the development of eosinophilic inflammation of the middle ear. When studying the auditory tube function using tubosonometry in 20 patients with EOM, signs of its gaping were revealed [14]. This facilitates the penetration of antigens and cytokines into the tympanic cavity with the subsequent development of eosinophilic inflammation [14, 15].
A histological examination of the mucous membrane of the middle ear reveals signs of chronic inflammation with infiltration by eosinophils and plasma cells with a small number of neutrophils [7, 16].
Examining the middle ear discharge Yukiko Iino and coauthors found a large number of activated cells (EG2-positive cells) among eosinophils. Many of them were degranulated and/or had signs of karyolysis/karyorexis and a high level of eosinophilic cationic protein and eosinophilic chemoattractants (IL-5, eotoxin). In the mucous membrane of the middle ear itself, the number of eosinophils is significantly lower, which indicates their directed migration to the middle ear cavity [16]. Subsequently, local cytolysis of eosinophils promotes the release of not only eosinophilic granules, but also extracellular DNA traps, which leads to exudate thickening.
Thus, the mechanism of programmed cell death, accompanied by the release of extracellular chromatin, the so-called extracellular chromatin trap—Ethosis plays the key role in the formation of viscous exudate in the middle ear cavity. A chromatin trap consists of a long DNA strand wrapping histone proteins in the form of “beads on a string” [17]. Ejected chromatin traps quickly accumulate surrounding particles (eosinophilic granules and microorganisms) due to hydrophilic compounds, thereby forming stable aggregates. Despite the fact that this process limits the spread of microorganisms, its hyper-purity contributes to an increase in the viscosity of the secretion, which is observed in patients with EOM [18, 19].
When comparing middle ear discharge of EOM patients and otitis media of a different origin, one can find a high concentration of eosinophilic cationic protein (ECP) and EG2—positive cells in the EOM cases, which indicates the activity of eosinophilic inflammation. The concentrations of chemoattractants, IL-5, and eotoxin are significantly higher in EOM patients and correlates positively with the concentration of eosinophilic cationic protein (ECP). Also, it positively correlates with the level of interleukin 5. This chemoattractant is involved not only in the migration of eosinophils, but also in their activation and increases the lifespan of these cells. The authors note that IL-5 is a key link in the pathogenesis of the development of EOM, as it stimulates the migration and local activation of eosinophils and prolonging the cell cycle [16].
Immunohistochemical analysis reveals that the number of cells immunopositive for IL-5 and ecalectin, which are chemoattractants and activating factors for eosinophils, is much higher in the mucous membrane of the respiratory tract and ear in EOM. An increase in the neurotoxin of eosinophils, elastase, IL-2, MIP-1α, and IL-1ra was also observed in the mucous membrane of the middle ear of EOM patients [20].
The fact that chemoattractants and eosinophil activators were found not only at the protein level, but also at the level of RNA messenger, indicates the active eosinophilic inflammation in the mucous membrane of the middle ear [16, 21]. A significant level of IgE (presumably of local origin), many times higher than the concentration of IgE in blood plasma, is also determined in middle ear effusion [22,23,24].
The level of IgE in middle ear effusion in EOM patients positively correlates with the level of sensorineural hearing loss, namely with a decrease in bone conduction at 2 and 4 kHz. Local excessive production of IgE in the middle ear ultimately leads to inner ear damage [16].
Matsubara et al. undertook an experimental study on guinea pigs to determine the mechanism of inner ear structures damage in response to eosinophilic inflamation. Experimental animals underwent general peritoneal and local intrathympanic sensitization with ovalbumin (egg white albumin) followed by histological and immunohistochemical analysis of middle and inner ear tissues to assess the damage mechanism. Morphological and histochemical changes were recorded on the 7th, 14th and 28th days of the experiment. On the 28th day significant damage of the middle and inner ear was noted. Inner ear structures contained a vast amount of eosinophils, plasmocytes and lymphocytes. There were a significant number of eosinophils, lymphocytes, plasmocytes, neutrophils and macrophages in the scala tympany. Partial penetration of bacteria and neutrophils through the membrane of the cochlear window was marked. Moreover, there were destructive changes in the capillaries of the basement membrane, severe morphological damage of the Corti organ, the spiral ligament and vascular strip. The presence of nystagmus in several animals could indicate involvement of the vestibular part of the inner ear [25].
Penetration of eosinophils into the inner ear may also be a result of an allergic reaction. Against the background of sensitization with ovalbumin, tympanic epithelial cells begin to produce eotaxin-3 and cytokine A5. With the further development of the process, inflammatory cells such as lymphocytes, plasmocytes, eosinophils also become sources of the above-mentioned chemokines, which penetrate the round window membrane and lead to the migration of eosinophils into the inner ear [25, 26]. Cytotoxic proteins and reactive oxygen species produced by eosinophils lesion the epithelial layer of the round window membrane, increasing its penetrance. The overlay of a bacterial infection and the diffusion of bacterial toxins into the perilymph also contributes to Corti organ damage, causing the development of sensorineural hearing loss [16].
The determination of cytokines involved in the pathogenesis of EOM plays an important role not only in understanding the mechanisms of the disease, but also in the search of treatment methods, for example, the possibility of using targeted biological drugs.
Periostin was found in the basement membrane and extracellular matrix of patients with EOM. This protein is secreted by pulmonary fibroblasts and plays an important role in the development of subepithelial fibrosis in bronchial asthma [27]. Isido et al. observed the production of periostin in nasal polyps in a number of patients, including concomitant BA, as well as in patients with the aspirin triad. Periostin promotes tissue fibrosis and remodeling of the nasal mucosa [28]. According to the authors, periostin plays an important role in the formation of granulations in the middle ear in EOM [29].
Clinical Features
In 2011a multicenter study was conducted in five japanese clinical centers, to analyse the observation results of 138 EOM patients. As a result, the clinical features of this disease were evaluated and the following diagnostic criteria were proposed (Table 1) [9].
The main clinical signs of EOM are [30]:
-
1.
Bilateral nature of the lesion
-
2.
Visualization of large granulations in the tympanic cavity during perforation of the tympanic membrane; if there is no perforation, then the swelling of the eardrum is yellow
-
3.
Concomitant polypous rhinosinusitis
-
4.
The presence of sensorineural hearing loss
-
5.
Aerial attic and antrum according to CT data
-
6.
Ineffectiveness of standard conservative treatment regimens for exudative/chronic otitis media
-
7.
Improving the condition of the middle ear after applying systemic/local glucocorticosteroids.
The clinical features of the middle ear condition in EOM can be divided into 2 types: otitis media with effusion symptoms or signs of chronic otitis media with the presence of constant perforation. Despite the presence or absence of perforation of the tympanic membrane in all cases of EOM, a particularly viscous exudate is noted in the tympanic cavity. When installing a transtimpanal shunt, the lumen of the tube is very easily blocked by the viscous discharge from the middle ear.
Iino et al. proposed a scale for assessing the severity of the of EOM according to the middle ear mucous membrane condition, taking into account the following criteria (Table 2).
Kanazawa evaluated the severity of the aforementioned symptoms in 26 patients every 3 months. Having obtained the average score for 4 three-month periods (1 year), the authors analyzed the influence of the following parameters on the severity of the course of EOM—gender, age, body mass index (BMI), duration of bronchial asthma, relationship with aspirin-dependent bronchial asthma, the sum of the points on a Lund—Mackay scale, the degree of mastoid process pneumatization, the width of the bony part of the auditory tube in the area of the tympanic opening, the values of eosinophils and peripheral blood IgE, the relationship with allergic rhinitis. As a result of the analysis of the created model of multiple linear regression, it was found that excessive BMI and the duration of the course of bronchial asthma (the period between the first episode and the first visit to the clinic) significantly aggravate the course of EOM (p < 0.05) [31].
Bronchial asthma occurs in 90% of cases of EOM and EOM occurs in 10% of BA patients. The relationship between these two pathological processes can be combined into “one airway, one disease” theory. The middle ear cavity, connected with the nasopharynx through the Eustachian tube, can be considered as part of the respiratory tract [4]. Thus, the optimal treatment of bronchial asthma influence the condition of the middle ear in EOM patients. With adequate treatment of bronchial asthma, after selecting a therapy that allows to achieve the stable remission, the condition of the middle ear improves, the otorrhea disappears, the swelling of the mucous membrane decreases, granulation disappears, which, in turn, leads to better hearing [32, 33].
Diagnosis: Examination Technics
The examination scheme for patients with middle ear pathology, in addition to the standard otorhinolaryngological examination, includes audiological examination, as well as computed tomography (CT), (in rare cases, magnetic resonance imaging (MRI)) of the temporal bones.
Mixed hearing loss is usually observed on the audiograms of EOM patients. The incidence and severity of sensoneural hearing loss in EOM is significantly higher compared with otitis media of another etiology. In the late stages of the disease, patients have bilateral hearing loss with a low degree of bone conduction. Thus, in the course of the disease, conductive hearing loss progresses to sensorineural up to complete deafness [16].
There is very little data in the literature on the X-ray examination results in EOM patients. CT-scans usually shows the filling of the middle ear and mastoid with a soft tissue component. On MRI the soft-tissue mass gives a weak signal in T1 mode and a medium-intense in T2 mode.
Thus, the picture of CT and MRI in EOM is not specific. The following signs may indicative in favour of EOM [34]:
-
1.
Bilateral involvement
-
2.
Signs of rhinosinusitis (thickening of the mucosa of the maxillary sinuses)
-
3.
Patulous Eustachian tubes
Additionally, the diagnostic criteria for EOM require an eosinophilrich middle ear effusion confirmed morphologically. The quality of the morphologic findings is often suboptimal because of the high effusion viscosity. Some authors propose the flow cytometry as a diagnostic tool, which enables to quantificate eosinophils, redefine the morphology, and estimate the response to therapy [35].
Differential Diagnostics
It is necessary to exclude Charge-Strauss syndrome and hypereosinophilic syndrome in the presence of EOM symptoms.
Eosinophilic granulomatosis with polyangiitis (EGPA), also known as Churg–Strauss syndrome is a rare autoimmune disease characterized by to several organs damage due to the development of eosinophilic infiltration and vasculitis. This disease is often combined into one group with Wegener’s granulomatosis due to the frequent appearence of antineutrophilic cytoplasmic antibodies (ANCA) in the blood, however, a distinctive feature of the Charge-Strauss syndrome is an allergic background, which manifests itself in an increase of blood eosinophils, the development of bronchial asthma, as well as eosinophilic inflammation of the upper respiratory tract. Pathology can develop at any age, but the peak incidence occurs at the age of 50–60 years [36]. Currently there are no clear diagnostic criteria for diagnosing Charge-Strauss syndrome. Chapel Hill Consensus (international conferences which addressed the need of standardized classification system for systemic vasculities) defines it as eosinophil-rich granulomatous inflammation of the airways and necrotic vasculitis affecting small and medium vessels associated with asthma and eosinophilia [37].
In 1990, the American College of Rheumatology (ACR) established that at least four of the
following six criteria should be fulfilled to diagnose EGPA [35]:
-
1.
A history of asthma
-
2.
Eosinophilia > 10%,
-
3.
Mononeuropathy or polyneuropathy
-
4.
Nonfixed pulmonary infiltrates
-
5.
Paranasal sinus abnormalities
-
6.
Biopsy showing extravascular eosinophils
Otological manifestations are not included in the criteria. However, there are reports of middle and inner ear involvement in the literature, presented by hearing loss, chronic otorrhea with viscous effusion and chronic granulomatous eosinophilic infiltrates in the tympanic cavity and mastoid process [38,39,40]. The etiology of hearing loss in the case of Charge-Strauss syndrome can be both a consequence of the development of eosinophilic inflammation in the middle ear, and the result of ischemic neuritis.
Due to the analysis of the symptoms of 34 patients with EGPA, otitis media with viscous effusion was presented in 17.2% cases and 52% of patients had hearing loss (mostly conductive).
The otologic symptoms occurred before EGPA diagnosis [41].
Differential diagnosis of EOM and Charge-Strauss syndrome is extremely important, as patients with the syndrome should receive systemic treatment using glucocorticosteroids and cytostatics to prevent life-threatening complications, such as, for example, cardiomyopathy [42].
First, the doctor should pay attention to the presence of neurological disorders or a characteristic rash, indicating the presence of vasculitis.
Also, patients should be tested for antineutrophil antibodies, ANCA (MPO and P3). The Japanese Otological Society has proposed the concept of an early Charge-Strauss syndrome diagnosis, based on a combination of eosinophilic otitis media and ANCA-associated vasculitis [38]. Unfortunately, ANCA is detected in 30-70% of patients with Charge Strauss syndrome [43]; therefore, a number of authors suggest long-term follow-up of patients with EOM due to the risk of the Charge-Strauss syndrome [44].
Hypereosinophilic syndrome is a disease characterized by a constant idiopathic increase of eosinophils in blood (≥ 1500 eosinophils/mm3) for at least 6 months. The heart, nervous system, bone marrow, blood vessels, lungs, and skin may be involved in the pathological process [45]. In 1995 the possible involvement of the middle ear with the development of EOM was described [46]. The diagnosis of hypereosinophilic syndrome is based on exclusion of clonal and reactive eosinophilia (response to infection, autoimmune disease, atopy, hypoadrenalism, or oncology), as well as tropical eosinophilia. If untreated (glucocorticoids with a possible combination with monoclonal antibodies), hypereosinophilic syndrome worsens and leads to death [47, 48].
Treatment
Until recently, the treatment of patients with EOM was based on the use of glucocorticosteroids more often topical, less often systemic [5, 7, 30, 49]. According to Suzuki H. analysis of the treatment of 340 EOM patients, local and systemic steroids were used in 78% and 66% of patients, respectively. The ratio of sensorineural hearing loss was significantly higher in patients who were given systemic steroids than in patients without systemic steroids (52% vs. 34%, p < 0.05) [3].
Intrathimpanic administration of glucocorticosteroids is crucial for maintaining the function of the inner ear [50]. It is equally important,to remove rich in eosinophils and inflammatory mediators exudate from the tympanic cavity as soon as possible. To achieve this, a myringotomy is performed, as well as various shunt procedures. Heparin can be used to dilute and facilitate the removal of viscous effusion. Along with that heparin inhibits the chemotaxis of eosinophils, as well as cationic proteins of eosinophilic granules [51]. In case of bacterial inflammation it is possible to use local antibacterial drugs.
The surgical treatment, such as antromastoidotomy, tympanoplasty is unacceptable for this group of patients, because it leads to the exacerbation of sensorineural hearing loss. The analysis of the treatment methods of 340 patients showed, that only in 13% tympanoplasty was performed. At the same time, significant sensorineural-type hearing loss was recorded in 17% of patients after surgery, and only in 4% without surgical treatment [15].
Yoshihiko et al. evaluated the efficacy of treatment for patients with EOM. The authors identified 3 degrees of the middle ear mucous membrane condition: 1 degree (G1)—unchanged mucous membrane, 2 degree (G2)—thickened mucous membrane of the middle ear, 3 degree (G3)—thickened mucosa with granulations that extend beyond tympanic cavity.
It was found that topical treatment (introduction of triamcinolone into the tympanic cavity) is effective in G1, with G2 additional systemic use of glucocorticosteroids is necessary. G3 changes were observed in case of bacterial inflammation overlay, which required the addition of antibiotic therapy. Local treatment in G3 was effective only after surgical removal of granulation tissue [52].
Along with local treatment, it is very important to conduct adequate therapy for BA. According to observations, when the exacerbation is relieved and the intensity of pulmonary symptoms decreases, the condition of the middle ear also improves [53].
Attempts are currently underway to treat EOM with targeted monoclonal antibodies, known as biologicals. These include lebricizumab (anti-IL-13), reslizumab (anti-IL-5), dupilumab (anti-IL 4Ra), mepolizumab (anti-IL-5), omalizumab (anti-IgE) and beralizumab (anti-IL) -5R). These drugs have been shown to be effective in treating patients with CRSwNP. According to the results of clinical trials, use of mepolizumab (n = 30) and reslizumab (n = 24) has lead to a decrease of nasal polyps compared to the control group [54]. Based on the results of two studies (n = 724), Dupilumab was recently approved by the US Food and Drug Administration (FDA) as the first drug for treating patients with ChRSwithP [55].
In pilot studies Iino et al. demonstrated the efficacy of mepolizumab as an additional treatment for corticosteroid therapy in 13 patients with EOM. After 3 months of therapy, patients taking mepolizumab showed a significant decrease in the EOM symptoms intensity, compared to the control group. After a year of mepolizumab using, almost all patients noted a steady improvement of middle ear condition. Besides a significant decrease in peripheral blood eosinophilia was observed. The therapy was ineffective only in two patients, who had granulations in the tympanic cavity. That enabled the authors to conclude that the use of monoclonal antibodies in the presence of granulation tissue in the tympanic cavity is ineffective. It is important to note that sensonueral –type hearing impairment was more often observed in the control group [56].
Omalizumab was also effective and reduced eosinophilic inflammation in the middle ear. After 3 months of omalizumab administration eosinophil cationic protein concentrations in middle ear effusion significantly decreased [57,58,59].
The literature describes cases of successful use of an inhibitor of IL-5R beralizumab in the treatment of patients with EOM. First approved by the US Food and Drug Administration in 2017 for the treatment of severe cases of eosinophilic asthma, beralizumab is highly effective in lowering blood eosinophils [60]. Further cases of successful treatment of patients with EOM using a thromboxane A2 antagonist, ramatroban are described in literature [61]. A recent study showed the efficacy of pegylated interferon-2a and 2b in such patients, but with frequent development of side effects, including myalgia and fatigue [6].
Thus, further case studies and prospective trials are needed to evaluate the efficacy of benralizumab and other monoclonal antibodies as an independent therapy for EOM.
The importance of early diagnosis and the need for adequate treatment in EOM is due to the fact that the progression of the disease leads to the development of not only conductive, but also sensorineural hearing loss [62], which is associated with the progression of eosinophilic inflammation and bacterial infection of inner ear. The described above studies report that approximately 50% of previously untreated patients with EOM had a decrease in bone conduction, and in 6%—complete deafness [31].
Such patients need hearing rehabilitation. In 2006, Iwasaki et al. published data on the successful cochlear implantation of a patient with EOM. The surgery has its own peculariries, since the installation of a foreign body in the structures of the ear can lead to the progression of eosinophilic inflammation. In addition, the ongoing eosinophilic inflammation in the middle ear, the formation of viscous exudate leads to the need for shunting of the tympanic cavity, which, in turn, increases the risk of meningitis. Therefore, in case of cochlear implantation of EOM patients some authors suggest to perform subtotal petrosectomy with removal of the mucous membrane of the tympanic cavity, all mastoid cells, blocking the auditory tube and filling the cavity with adipose tissue. In the preoperative period, a course of systemic steroid therapy is recommended to a decrease in the volume of granulations and edematous mucous membrane and thus to reduce intraoperative bleeding. [63, 64].
EOM is a relatively rare pathology, however the incidence of the disease seems to be underestimated due to the lack of diagnostic tools. Further studies of the prevalence, clinical features, pathogenesis and treatment tools of this pathological entity as well as the issues of rehabilitation of EOM patients are necessary.
References
Koch H (1947) Allergical investigations of chronic otitis. Acta Otolaryngol 62(Suppl.):1–201
Tomioka S, Yuasa R, lino Y (1994) Intractable otitis media in cases with bronchial asthma. Recent advances in otitis media with effusion. In: Proceedings of the second extraordinary international symposium on recent advances on otitis media. Kugler Publications, Inc., Amsterdam, pp 183–186
Suzuki H, Matsutani S, Kawase T, Iino Y, Kawauchi H, Gyo K et al (2004) Epidemiologic surveillance of ‘‘eosinophilic otitis media’’ in Japan. Otol Jpn 14:112–117 (In Japanese)
Grossman J (1997) One airway, one disease. Chest 111(2 Suppl):11S–16S. https://doi.org/10.1378/chest.111.2_supplement.11s
Azadarmaki R, Westra W, Prasad S (2015) Eosinophilic Mucin otomastoiditis and otopolyposis: a progressive form of eosinophilic otitis media. Ann Otol Rhinol Laryngol 124(9):752–756. https://doi.org/10.1177/0003489415577988
Neff BA, Voss SG, Carlson ML, O’Brien EK, Butterfield JH (2017) Treatment of eosinophilic otitis media with pegylated interferonalpha 2a and 2b. Laryngoscope 127:1208–1216
Lara-Sánchez H, Vallejo L (2017) Eosinophilic otitis media. N Engl J Med 376(7):e10. https://doi.org/10.1056/NEJMicm1510852
Chow K, Cosetti M (2020) Use of IL-5 inhibitor benralizumab as a novel therapy for eosinophilic otitis media. Otol Neurotol 41(2):e238–e240. https://doi.org/10.1097/MAO.0000000000002493
Iino Y, Tomioka-Matsutani S, Matsubara A, Nakagawa T, Nonaka M (2011) Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx 38:456–461
Omori M, Ziegler S (2007) Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol 178(3):1396–1404. https://doi.org/10.4049/jimmunol.178.3.1396
Simson L, Foster PS (2000) Chemokine and cytokine cooperativity: eosinophil migration in the asthmatic response. Immunol Cell Biol 78(4):415–422. https://doi.org/10.1046/j.1440-1711.2000.00922.x
Yao T, Kojima Y, Koyanagi A et al (2009) Eotaxin-1, -2, and -3 immunoreactivity and protein concentration in the nasal polyps of eosinophilic chronic rhinosinusitis patients. Laryngoscope 119(6):1053–1059. https://doi.org/10.1002/lary.20191
Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, Honda K, Ohta N, Yamamoto S, Takagi Y, Wada A, Weller PF (2016) Eosinophil extracellular trap cell death-derived DNA traps: their presence in secretions and functional attributes. J Allergy Clin Immunol 137(1):258–267. https://doi.org/10.1016/j.jaci.2015.04.041Epub 2015 Jun 9
IinoY Kakizaki K, Saruya S, Katano H, Komiya T, Kodera K et al (2006) Eustachian tube function in patients with eosinophilic otitis media associated with bronchial asthma evaluated by sonotubometry. Arch Otolaryngol Head Neck Surg 132:1109–1114
Kanazawa H, Yoshida N, Hara M, Hasegawa M, Matsuzawa S, Shinnabe A et al (2013) Risk factors for eosinophilic otitis media in patients with eosinophilic chronic rhinosinusitis. Int Adv Otol 9:353–358
Iino Y (2010) Role of IgE in eosinophilic otitis media. Allergol Int 59:233–238. https://doi.org/10.2332/allergolint.10-RAI-0223
Ohta N, Ueki S, Konno Y, Hirokawa M, Kubota T, Tomioka-Matsutani S, Suzuki T, Ishida Y, Kawano T, Miyasaka T, Takahashi T, Suzuki T, Ohno I, Kakehata S, Fujieda S (2018) ETosis-derived DNA trap production in middle ear effusion is a common feature of eosinophilic otitis media. Allergol Int 67(3):414–416. https://doi.org/10.1016/j.alit.2017.11.007
Ueki S, Ohta N, Takeda M, Konno Y, Hirokawa M (2017) Eosinophilic otitis media: the aftermath of eosinophil extracellular trap cell death. Curr Allergy Asthma Rep 17(5):33. https://doi.org/10.1007/s11882-017-0702-5
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191(3):677–691. https://doi.org/10.1083/jcb.201006052
Uchimizu H, Matsuwaki Y, Kato M, Otori N, Kojima H (2015) Eosinophil-derived neurotoxin, elastase, and cytokine profile in effusion from eosinophilic otitis media. Allergol Int 64(Suppl):S18–S23. https://doi.org/10.1016/j.alit.2015.03.007Epub 2015 Apr 25
Miura T, Matsubara A, Kudo N, Hara R, Takahata J, Sasaki A (2018) The expression of thymic stromal lymphopoietin in patients and animal models with eosinophilic otitis media. Acta Otolaryngol 138(5):447–451. https://doi.org/10.1080/00016489.2017.1416170
Nonaka M, Fukumoto A, Ozu C, Mokuno E, Baba S, Pawankar R et al (2003) IL-5 and eotaxin levels in middle ear effusion and blood from asthmatics with otitis media with effusion. Acta Otolaryngol 123:383–387
Iino Y, Kakizaki K, Katano H, Saigusa H, Kanegasaki S (2005) Eosinophil chemoattractant in middle ear patients with eosinophilic otitis media. Clin Exp Allergy 35:1370–1376
Iino Y, Nagamine H, Yabe T, Matsutani S (2001) Eosinophils are activated in middle ear mucosa and middle ear effusion of patients with intractable otitis media associated with bronchial asthma. Clin Exp Allergy 31:1135–1143
Matsubara A, Nishizawa H, Kurose A, Nakagawa T, Takahata J, Sasaki A (2014) An experimental study of inner ear injury in an animal model of eosinophilic otitis media. Acta Otolaryngol 134(3):227–232. https://doi.org/10.3109/00016489.2013.859395(Epub 2013 Dec 23)
Kudo N, Matsubara A, Nishizawa H, Miura T (2017) Immunohistological analysis of eotaxin and RANTES in the model animal of eosinophilic otitis media. Acta Otolaryngol 137(5):476–481. https://doi.org/10.1080/00016489.2016.1266508(Epub 2016 Dec 16)
Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S et al (2006) Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13-signals. J Allergy Clin Immunol 118:98–104
Okamoto M, Hoshino T, Kitasato Y, Sakazaki Y, Kawayama T, Fujimoto K et al (2011) Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J 5:1119–1127
Nishizawa H, Matsubara A, Nakagawa T, Ohta N, Izuhara K, Shirasaki T, Abe T, Takeda I, Shinkawa H (2012) The role of periostin in eosinophilic otitis media. Acta Otolaryngol 132(8):838–844. https://doi.org/10.3109/00016489.2012.668708Epub 2012 Jun 5
Iino Y (2008) Eosinophilic otitis media: a new middle ear disease entity. Curr Allergy Asthma Rep 8(6):525–530
Kanazawa H, Yoshida N, Yamamoto H, Hara M, Hasegawa M, Matsuzawa S, Shinnabe A, Iino Y (2014) Risk factors associated with severity of eosinophilic otitis media. Auris Nasus Larynx 41(6):513–517. https://doi.org/10.1016/j.anl.2014.08.003
Tanaka Yukako, Nonaka Manabu, Yamamura Yukie, Tagaya Etsuko, Pawankar Ruby, Yoshihara Toshio (2013) Improvement of eosinophilic otitis media by optimized asthma treatment. Allergy Asthma Immunol Res 5(3):175–178
Seo Yukako, Nonaka Manabu, Yamamura Yukie (2018) Optimal control of asthma improved eosinophilic otitis media. Asia Pacific Allergy 8(1):e5
Chung WJ, Lee JH, Lim HK, Yoon TH, Cho KJ, Baek JH (2012) Eosinophilic otitis media: CT and MRI findings and literature review. Korean J Radiol 13(3):363–367. https://doi.org/10.3348/kjr.2012.13.3.363
Saliba I, Alzahrani M, Weng X, Bestavros A (2017) Eosinophilic otitis media diagnosis using flow cytometric immunophenotyping. Acta Oto-Laryngol. https://doi.org/10.1080/00016489.2017.1385845
Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP et al (1990) The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 33:1094–1100
Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL et al (1994) Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 37:187–192
Harabuchi Yasuaki, Kishibe Kan, Tateyama Kaori, Morita Yuka, Yoshida Naohiro, Kunimoto Yasuomi, Matsui Takamichi, Sakaguchi Hiroshi, Okada Masahiro, Watanabe Takeshi, Inagaki Akira, Kobayashi Shigeto, Iino Yukiko, Murakami Shingo, Takahashi Haruo, Tono Tetsuya (2017) Clinical features and treatment outcomes of otitis media with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (OMAAV): a retrospective analysis of 235 patients from a nationwide survey in Japan. Mod Rheumatol 27(1):87–94. https://doi.org/10.1080/14397595.2016.1177926
Ishiyama A, Canalis RF (2001) Otological manifestations of Churg–Strauss syndrome. Laryngoscope 111(9):1619–1624. https://doi.org/10.1097/00005537-200109000-00024
Martinez Del Pero M, Moffat D, Sudhoff H (2008) Unusual presentation of temporal bone involvement in Churg–Strauss syndrome. J Laryngol Otol 122:425–427
Seccia V, Fortunato S, Cristofani-Mencacci L et al (2016) Focus on audiologic impairment in eosinophilic granulomatosis with polyangiitis. Laryngoscope 126(12):2792–2797. https://doi.org/10.1002/lary.25964
Ovadia S, Dror I, Zubkov T, Tanay A, Levy D, Zandman-Goddard G (2009) Churg–Strauss syndrome: a rare presentation with otological and pericardial manifestations: case report and review of the literature. Clin Rheumatol 28(Suppl. 1):S35–S38
Groh M, Pagnoux C, Baldini C et al (2015) Eosinophilic granulomatosis with polyangiitis (Churg–Strauss) (EGPA) Consensus Task Force recommendations for evaluation and management. Eur J Intern Med 26:545–553
Fukuda A, Morita S, Nakamaru Y, Hoshino K, Fujiwara K, Homma A (2019) Differentiation between eosinophilic otitis media and otitis media associated with eosinophilic granulomatosis with polyangiitis. Otol Neurotol 40(8):e796–e802. https://doi.org/10.1097/MAO.0000000000002295
Chusid MJ, Dale DC, West BC, Wolff SM (1975) The hypereosinophilic syndrome: analysis of fourteen cases with review of the literature. Medicine 54(1):1–27. https://doi.org/10.1097/00005792-197501000-00001
Takayama K, Yadohisa O, Furuno T, Hashimoto S, Nakanishi Y, Abe M et al (1995) Case report: the first report of idiopathic hypereosinophilic syndrome involved with lung and middle ear. Am J Med Sci 309:282–284
Reiter A, Gotlib J (2017) Myeloid neoplasms with eosinophilia. Blood 129(6):704–714. https://doi.org/10.1182/blood-2016-10-695973
Longmore M, Wilkinson I, Turmezei T, Cheung CK (2007). Oxford handbook of clinical medicine. Oxford. p 316. ISBN:978-0-19-856837-7
Iino Y, Nagamine H, Kakizaki K, Komiya T, Katano H, Saruya S et al (2006) Effectiveness of instillation of triamcinolone acetonide into the middle ear for eosinophilic otitis mediaassociated with bronchial asthma. Ann Allergy Asthma Immunol 97:761–766
Iino Y, Nagamine H, Kakizaki K et al (2006) Effectiveness of instillation of triamcinolone acetonide into the middle ear for eosinophilic otitis media associated with bronchial asthma. Ann Allergy Asthma Immunol 97(6):761–766. https://doi.org/10.1016/S1081-1206(10)60967-2
Matsubara A, Kuroda R, Usami S, Takahata J, Shinkawa H (2001) The effect of topical administration of heparin to the eosinophilic otitis media. In: Takasaka T, Yuasa R, Hozawa K (eds) Recent advances in otitis media. Bologna: Monduzzi Editore, pp 403–406
Esu Y, Iino Y, Masuda M, Kanazawa H, Yoshida N (2018) Proposal of a treatment strategy for eosinophilic otitis media based on middle ear condition. Otol Neurotol 39(8):e671–e678. https://doi.org/10.1097/MAO.0000000000001912
Okude A, Tagaya E, Kondo M, Nonaka M, Tamaoki J (2012) A case of severe asthma with eosinophilic otitis media successfully treated with anti-IgE monoclonal antibody omalizumab. Case Rep Pulmonol 2012:340525
Smith KA, Pulsipher A, Gabrielsen DA, Alt JA (2018) Biologics in chronic rhinosinusitis: an update and thoughts for future directions. Am J Rhinol Allergy 32:412–423
FDA approves first treatment for chronic rhinosinusitis with nasal polyps [news release]. Silver Spring, MD: Food and Drug Administration Office of Media Affairs; 2019. Avaliable at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatmentchronic-rhinosinusitis-nasal-polyps. Accessed July 2 2019
Iino Y, Takahashi E, Ida S, Kikuchi S (2019) Clinical efficacy of anti-IL-5 monoclonal antibody mepolizumab in the treatment of eosinophilic otitis media. Auris Nasus Larynx 46(196–203):15
Iino Y, Hara M, Hasegawa M et al (2012) Clinical efficacy of anti-IgE therapy for eosinophilic otitis media. Otol Neurotol 33:1218–1224
Iino Y, Hara M, Hasegawa M et al (2014) Effect of omalizumab on biomarkers in middle ear effusion in patients with eosinophilic otitis media. Acta Otolaryngol 134(4):366–372. https://doi.org/10.3109/00016489.2013.868601
El-Qutob D (2016) Off-label uses of omalizumab. Clinic Rev Allerg Immunol 50:84–96. https://doi.org/10.1007/s12016-015-8490-y
Markham A (2018) Benralizumab: first global approval. Drugs 78:505–511. https://doi.org/10.1007/s40265-018-0876-8
Wada T, Uemaetomari I, Murashita H et al (2006) Successful treatment of eosinophilic otitis media using ramatroban: report of two cases. Auris Nasus Larynx 33(4):455–460. https://doi.org/10.1016/j.anl.2006.05.007
Iino Y, Usubuchi H, Kodama K et al (2010) Eosinophilic inflammation in the middle ear induces deterioration of bone-conduction hearing level in patients with eosinophilic otitis media. Otol Neurotol 31:100–104
Iwasaki S, Nagura M, Mizuta K (2006) Cochlear implantation in a patient with eosinophilic otitis media. Eur Arch Otorhinolaryngol 263:365–369. https://doi.org/10.1007/s00405-005-1006-2
Sugimoto H, Hatano M, Noda M et al (2017) Cochlear implantation in deaf patients with eosinophilic otitis media using subtotal petrosectomy and mastoid obliteration. Eur Arch Otorhinolaryngol 274:1173–1177. https://doi.org/10.1007/s00405-016-4091-5
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This article contains review of literary data. We havn’t made any research including human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shevchik, E., Svistushkin, V., Nikiforova, G. et al. Eosinophilic Otitis Media: Modern Aspects of Pathogenesis, Clinical Features, Diagnosis and Treatment. Indian J Otolaryngol Head Neck Surg 74 (Suppl 1), 132–140 (2022). https://doi.org/10.1007/s12070-020-01903-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-020-01903-z