Abstract
Cetuximab (EGFR-targeted IgG1 monoclonal antibody) has shown to improve the treatment outcomes in head and neck cancer. The evidence on the beneficial outcomes of cetuximab with radiotherapy (RT) in unresectable patients of locally advanced squamous cell carcinoma of head and neck (LA-SCCHN) is limited in real-life practice. The present study evaluated the treatment outcomes of cetuximab concurrent with RT in Indian patients with unresectable LA-SCCHN. We retrospectively reviewed fifty-one patients with unresectable LA-SCCHN between January 2013 and December 2017, who were treated with cetuximab concurrently with RT. Tumor response and disease-free survival (DFS) were estimated. Tumor response using RECIST (1.1) criteria reported complete response in 66.7%, partial response in 31.4% and progressive disease in 1.9% of the patients. The overall response rate was 98%. The 1-year and 2-year DFS was 85% and 69% respectively. The median DFS was significantly better in stage 3 than stage 4. The most common toxicity observed was mucositis and skin reactions (grade 3). Cetuximab concurrent with RT was effective in Indian patients with unresectable, LA-SCCHN and had an acceptable toxicity profile in real-life practice. The real-life beneficial evidence of the combination is consistent with the results documented in the randomized controlled trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Squamous cell carcinoma of the head and neck (SCCHN), including the oral cavity, nasopharynx, hypopharynx, larynx and tongue, is the most common cancer globally [1]. They account for more than half million new cases and 380,000 deaths per year [2]. Major etiological risk factor includes tobacco use, betel-quid and Areca-nut chewing, alcohol consumption, human papilloma virus infection, and Epstein–Barr virus infection [3]. In the Indian subcontinent, head and neck cancers are the most common cancers [1]. There is a rising burden of SCCHN owing to high prevalence of tobacco (including smokeless tobacco) and Areca-nut chewing habit and the majority present in locally advanced stages [4,5,6].
The management of locally advanced SCCHN (LA-SCCHN) consists of surgery, radiation, and chemotherapy. Traditionally, surgery followed by adjuvant therapy has been the most common treatment standard for LA-SCCHN [7, 8]. However, in unresectable patients of LA-SCCHN, chemoradiotherapy (CRT) or radiotherapy (RT)—based modality is the primary standard [8].
With current levels of cumulative evidence on beneficial outcomes, definitive concurrent platinum-based CRT has emerged as the contemporary standard of care for unresectable LA-SCCHN, but is hampered by its immediate and late toxicity profile [9, 10]. Moreover, a substantial subset of LA-SCCHN patients are often unsuitable for platinum-based therapy and being treated with radiotherapy alone with suboptimal outcomes [10, 11]. Thus, there is a pressing need for efficacious alternatives to platinum-based therapy that can be combined with RT to improve outcomes.
Epidermal growth factor receptor (EGFR) is abnormally activated and overexpressed in more than 90% of SCCHN [12, 13]. Elevated EGFR is associated with oncogenesis and is an independent predictor of poor prognosis in SCCHN [7, 13,14,15,16]. Radiation exposure tends to increases the expression of EGFR in tumor cells, and blocking EGFR signaling sensitizes tumor cells to the effects of radiation [7, 11, 17, 18]. Thus, EGFR-based targeted inhibitors represent a potential novel strategy to achieve improved outcomes compared to RT alone.
Cetuximab is a chimeric immunoglobulin G1 monoclonal antibody that specifically targets EGFR with high affinity, inhibiting endogenous ligand binding with resultant blockade of receptor dimerization, tyrosine kinase phosphorylation and signal transduction [7, 19, 20]. Moreover, cetuximab induces cell-mediated cytotoxicity and sensitizes tumor cells to the effects of radiation in SCCHN [7, 20,21,22].
The efficacy of cetuximab in LA-SCCHN has been validated in the landmark Bonner et al. trial [7]. Bonner trial was a randomized, phase -3 study conducted across 73 centers in the United States and 14 other countries, in which patients of LA-SCCHN were randomly assigned to RT plus concomitant cetuximab versus RT alone [7]. The trial demonstrated that RT plus concomitant cetuximab was superior to RT alone in terms of locoregional control, progression-free survival and 5-year overall survival [7, 23].
Based on the encouraging outcomes observed in Western population in the Bonner et al. trial [7], cetuximab with RT has been recommended in clinical guidelines for management of LA-SCCHN [24].
The evidence on the beneficial outcomes of cetuximab with RT in subset of Indian patients with unresectable LA-SCCHN is limited [11, 25] and there is a need to gather cumulative evidence on the beneficial outcomes achieved in real-life practice. We, therefore conducted this retrospective analysis to evaluate the treatment outcomes (tumor response rate and disease-free survival) of cetuximab concurrent with RT in unresectable LA-SCCHN patients at a tertiary care hospital in rural India.
Materials and Methods
In the present study, we retrospectively reviewed the medical records of newly diagnosed patients with unresectable LA-SCCHN who received cetuximab concurrent with radiotherapy between January 2013 and December 2017 at the Oncology department of a tertiary care hospital in Jabalpur (India). Data regarding patient characteristics, tumor site, tumor stage, investigations, clinical course with treatment modalities, imaging scan reports and treatment-related toxicities were collected.
The patients included in this retrospective analysis were selected based on the following: (a) histologically proven squamous cell carcinoma of head and neck, (b) locally advanced (stage 3 to 4b), (c) inoperable tumor, (d) age ≥ 18 years, (e) Karnofsky performance score ≥ 60, and (f) treated with RT (up to 70 Gy) plus cetuximab (loading dose 400 mg/m2 followed by 250 mg/m2 weekly) combination. We excluded patients of recurrent or metastatic head and neck cancer, previously treated chemotherapy or RT or both, had received any other anti-EGFR agents and previously undergone surgical intervention.
Treatment Evaluation
The tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) criteria at 4 weeks after completion of radiotherapy [26]. The tumor response assessed included complete response (CR), partial response (PR), progression of disease (PD), and stable disease (SD) based on imaging scans of Computed tomography (CT)/Magnetic resonance imaging (MRI). The objective response rate (ORR) was calculated.
Disease-free survival (DFS) was calculated from the date of completion of radiation therapy to the date of first detection of cancer recurrence (Patients who survived a definite period of time without evidence of disease after therapy). Comparison of DFS with respect to age, gender, tumor stage, and anatomical site was performed. Toxicities were assessed and graded as per Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [27].
Statistical Analysis
Statistical Package for the Social Sciences (IBM SPSS, Windows) software version 21.0 was used for statistical analyses. Data were anonymized for all statistical analyses. Descriptive statistics were used to summarize the data. Median disease-free survival was calculated using the Kaplan–Meier method and differences were compared using the log-rank test. Two-tailed P values less than 0.05 were considered statistically significant.
Results
Baseline Patient, Disease and Treatment Characteristics
We analyzed data for fifty one patients with unresectable, LA-SCCHN treated with cetuximab concurrent with RT between January 2013 and December 2017.
The patient, tumor and treatment characteristics are detailed in Table 1. The median age was 55 years (Inter quartile range 49, 55, 60) with 39 (76.5%) males and 12 (23.5%) females. Addiction with one/two substance, with tobacco and bidi was common. The majority (80.4%) patients had stage 3 cancer and oral cavity (68.6%) was the commonest anatomical sub-sites. Majority patients received radiation dose of 70 Gy and 7 cycles (injections) of cetuximab 250 mg/m2 weekly (after the initial loading dose of 400 mg/m2) (Table 1).
Treatment Outcomes
Tumor Response Rate
Tumor response using RECIST criteria documented CR in 66.7% of patients, PR in 31.4% and PD in 1.9% of patients. The ORR observed was 98% (Table 2).
Disease-Free Survival (DFS)
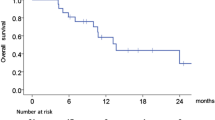
The Kaplan–Meier curves for DFS are shown in Fig. 1. The 1-year and 2-year DFS among all patients were 85% and 69% respectively (Fig. 1). The median follow-up was 20 months. The median DFS was not reached. On sub-group analysis based on tumor stage, the median DFS was significantly (P = 0.022) better in stage 3 than stage 4 (Fig. 2). The median DFS in patients with stage 4 was 21 months (95% CI 9.4–32.5), whereas in stage 3 the median DFS was not reached. However, no significant difference in median DFS was observed in subgroups defined by age, gender and anatomical site (P > 005).
Treatment Breaks and Follow-Up Duration
Majority patients had a treatment break of 1 week while the rest one-third patients had a break of 2 weeks. Majority patients were followed-up for 12 to 24 months of treatment (Table 1).
Recurrence and Metastasis
Around one-fourth (25.4%) of the total patients had recurrence of cancer, at an interval of 13.15 months. While only 4% had metastasis of cancer, at an interval of 11.5 months.
Safety and Toxicity
In this study, the most common toxicities encountered in all patients were grade 3 mucositis and grade 3 skin reactions. Non-hematological toxicity was seen more frequently than hematological toxicity. Neutropenia was observed in more than 50% of the patients but were of grade 1or 2 (Table 3). No grade 4 or anaphylactic reactions were observed in the study.
Discussion
The present retrospective study documented encouraging treatment outcomes and an acceptable degree of toxicity profile, suggesting that cetuximab concurrent with RT is a feasible option for Indian patients with unresectable, LA-SCCHN in real life practice.
The treatment of LA-SCCHN has evolved in recent years. Radiotherapy is the crucial treatment modality for unresectable SCCHN, administered alone or concurrent with chemotherapy [7, 8]. Concurrent platinum-based CRT is currently regarded as the leading standard of care for unresectable based on the robust evidences [9]. Unfortunately, the benefit is hampered due to toxicity burden and a significant proportion of patients are often unsuitable for platinum-based therapy (particularly patients with aged ≥ 70 years, low performance status, significant comorbidities, impaired kidney function and platinum drug allergy) [10, 11].
With the development of targeted therapies, especially cetuximab, there has been a shift in what is perceived as the optimal treatment approach in patients with unresectable LA-SCCHN. In 2006, a major advance in the treatment of LA-SCCHN was provided by phase 3 trial by Bonner et al. [7] that led to the regulatory approval of cetuximab plus RT combination. The trial reported that the addition of cetuximab to high-dose RT significantly improved loco-regional control (47% vs. 34%, hazard ratio: 0.68, 95% confidence interval [CI]: 0.52–0.89; P = 0.005) and overall survival (49 months vs. 29.3 months; hazard ratio: 0.74, 95% CI 0.57–0.97; P = 0.03) at 3-years as compared to RT alone with no appreciable increase in severe acute toxicity [7]. The trial also documented a significant difference in the best overall response favoring RT plus cetuximab combination compared to RT alone (74% vs. 64%; P = 0.02) [7].
The updated long term results (2010), confirmed the earlier findings of survival benefit with cetuximab plus RT combination (5-year OS: 49 months vs. 29.3 months; Hazard ratio: 0.73, 95% CI 0.56–0.95; P = 0.018) compared to RT alone [23].
Based upon the Bonner et al. trial in western populations, cetuximab plus RT has emerged a vital option for standard care as it offers a therapeutic alternative that is recognized to be superior to RT alone [7]. The tolerability and feasibility of cetuximab with RT has been evaluated in Japanese population with LA-SCCHN [28]. The tolerability and efficacy outcomes observed in Japanese population were in line with Bonner trial, despite the population diversity [28].
Currently, cetuximab with RT is recommended as category-1 evidence in the National Comprehensive Cancer Network (NCCN) guidelines in the management of LA-SCCHN [24]. In India, cetuximab-based therapy has been adopted into practice for the management of LA-SCCHN. There is limited data in the literature on the effectiveness of the combination in unresectable subset of patients from India. This retrospective study was important since it analyzed the real-world experiences of cetuximab concurrent with RT in Indian patients with unresectable LA-SCCHN from Rural parts of Central India.
In the present study, we documented that cetuximab concurrent with RT resulted in improved clinical outcomes; CR in 66.7% of patients, PR in 31.4% and PD in 1.9% of patients. The ORR achieved in this study was 98%. These results of improved response rate are in agreement, but more favorable than those reported (74%) in the landmark Bonner et al. trial [7]. This could be due to the fact that the majority of patients enrolled in our study were less than 65 years of age (median age 55 years), male gender and tumor site oral cavity. Interestingly, such factors were associated with improved benefits with cetuximab therapy [7]. Moreover, the total dose of radiation received and treatment compliance could have resulted in better treatment outcomes.
There have been limited prospective reports describing improved response rates with cetuximab concurrent with RT in LA-SCCHN [22,23,24,25, 28, 29]. Acevedo-Henao et al. demonstrated that ORR was 91% with a CR of 74% [29]. Whereas, in Japanese patients with LA-SCCHN, Okano et al. documented ORR of 82% with a CR of 41% [28].
In the Indian context, only two studies have assessed cetuximab concurrent with RT in LA-SCCHN [11, 25]. In a prospective study among unresectable patients, Dattatreya et al. reported an ORR of 68.42%, disease control rate of 89.47% and overall survival (OS) of 84% at two years [25]. Whereas in a retrospective cohort study in 37 patients unfit for platinum-based therapy, Agarwal et al. observed an ORR of 97% and CR in 47% [11]. The 2-year loco-regional control, disease-free survival and overall survival reported were 35.5%, 29.5%, and 44.4% respectively [11]. Majority (86%) completed the planned course of RT without any interruption and received 6 or more cetuximab cycles [11]. Thus, Agarwal et al. outlined that cetuximab concurrent with RT was a reasonable option in patients ineligible for platinum-based therapy [11].
Finally, the response rates observed (98%) in the present study was similar but relatively higher than the findings from several prospective as well as Indian studies [11, 25,26,27]. The possible explanation for an improved response rate observed may be due to the fact that EGFR is highly over expressed in SCCHN. The addition of cetuximab thus advantages the blocking of the EGFR receptor-dependent pathway and also radio-sensitizes tumor cells, thereby suppressing tumor growth [7, 19, 20].
In the present study, we observed that the 2-year DFS was 69% at a median follow-up of 20 months. The 2-year DFS appears to be higher than that previously published by Agarwal et al. [11]. This difference may be due to the fact that in Agarwal et al. [11], patients with major co-morbidities and advanced age were included. In the present study, we found that the median DFS was significantly better in stage 3 than stage 4 (P = 0.022) which was similar to the findings of Bonner et al. trial,7 in which patients with stage 3 had better clinical outcomes than stage 4. This merits for use of this combination in the early stage of the disease.
In the present study, the incidence of grade 3 mucositis and grade 3 skin reactions were common and seen in all the patients. The toxicity profile observed in our study was similar to previous studies [11, 25, 28, 29]. However, we did not observe any serious toxicity (grade 4 or 5).
It is interesting to note in updated analysis of the Bonner trial that the development of prominent cetuximab-induced rash (grade 2 or above) was associated with improved overall survival (P = 0.002) compared to mild (grade 1) or no rash [23]. However, this possible association of effectiveness is debatable. In the present study, we observed that majority patients received a radiation dose of 70 Gy and 7 cycles (injections) of cetuximab (weekly) therapy which is comparable to findings of previous studies [11, 25]. The compliance to treatment in this study was good despite the incidence of grade 3 skin reactions.
Taking into consideration all limitations pertaining to retrospective design, our results of cetuximab concurrent with RT in real-life practice appear to be comparable to the beneficial outcomes as reported in clinical trial [7] and past research [11, 25, 28, 29].
Our study has certain intrinsic limitations. The main limitation is its retrospective design. The clinical outcomes evaluated lacked survival estimates as the information was limited and short-interval. The research was conducted in a single-institute; thereby results may be difficult to extrapolate to broader population. A longer follow-up period is required to substantiate the long-term benefits.
Conclusion
In conclusion, cetuximab concurrent with RT was effective for Indian patients with unresectable LA-SCCHN and had an acceptable toxicity profile. The real-life beneficial evidence of the combination is consistent with the results documented in the randomized controlled trial and literature studies.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C et al (2017) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol 3:524–548
Sankaranarayanan R, Masuyer E, Swaminathan R et al (1998) Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res 18:4779–4786
Kumar A, Chakravarty N, Bhatnagar S et al (2019) Efficacy and safety of concurrent chemoradiotherapy with or without Nimotuzumab in unresectable locally advanced squamous cell carcinoma of head and neck: prospective comparative study—ESCORT-N study. South Asian J Cancer 8:108–111
Dayal PK, Mani NJ, Bhargava K (1978) Prevalence of oral cancer and precancerous lesions in ‘pan’/’supari’ chewers. Indian J Public Health 22:234–245
Tuljapurkar V, Dhar H, Mishra A et al (2016) The Indian scenario of head and neck oncology—challenging the dogmas. South Asian J Cancer 5:105–110
Bonner JA, Harari PM, Giralt J et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Culliney B, Birhan A, Young AV et al (2008) Management of locally advanced or unresectable head and neck cancer. Oncology (Williston Park) 22:1152–1161 (discussion 1162–1166, 1171–1172)
Pignon JP, le Maître A, Maillard E et al (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4–14
Ahn MJ, D’Cruz A, Vermorken JB et al (2016) Clinical recommendations for defining platinum unsuitable head and neck cancer patient populations on chemoradiotherapy: a literature review. Oral Oncol 53:10–16
Agarwal JP, Gupta T, Kalyani N et al (2011) Cetuximab with radiotherapy in patients with loco-regionally advanced squamous cell carcinoma of head and neck unsuitable or ineligible for concurrent platinum-based chemo-radiotherapy: ready for routine clinical practice? Indian J Cancer 48:148–153
Mendelsohn J, Baselga J (2003) Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 21:2787–2799
Zimmermann M, Zouhair A, Azria D et al (2006) The epidermal growth factor receptor (EGFR) in head and neck cancer: its role and treatment implications. Radiat Oncol 1:11
Dassonville O, Formento JL, Francoual M et al (1993) Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol 11:1873–1878
Ang KK, Berkey BA, Tu X et al (2002) Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 62:7350–7356
Eriksen JG, Steiniche T, Askaa J et al (2004) The prognostic value of epidermal growth factor receptor is related to tumor differentiation and the overall treatment time of radiotherapy in squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 58:561–566
Bonner JA, Maihle NJ, Folven BR et al (1994) The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int J Radiat Oncol Biol Phys 29:243–247
Liang K, Ang KK, Milas L et al (2003) The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys 57:246–254
Saleh MN, Raisch KP, Stackhouse MA et al (1999) Combined modality therapy of A431 human epidermoid cancer using anti-EGFr antibody C225 and radiation. Cancer Biother Radiopharm 14:451–463
Huang SM, Bock JM, Harari PM (1999) Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res 59:1935–1940
Milas L, Mason K, Hunter N et al (2000) In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res 6:701–708
Harari PM, Huang SM (2001) Head and neck cancer as a clinical model for molecular targeting of therapy: combining EGFR blockade with radiation. Int J Radiat Oncol Biol Phys 49:427–433
Bonner JA, Harari PM, Giralt J et al (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11:21–28
National Comprehensive Cancer Network (2019) Clinical practice guidelines in oncology, head and neck cancers, version 2.2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 26 Aug 2019
Dattatreya S, Goswami C (2011) Cetuximab plus radiotherapy in patients with unresectable locally advanced squamous cell carcinoma of head and neck region—a open labelled single arm phase II study. Indian J Cancer 48:154–157
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version1.1). Eur J Cancer 45:228–247
National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE). CTCAE version 4.0 (2010). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Accessed 10 Jan 2017
Okano S, Yoshino T, Fujii M et al (2013) Phase II study of cetuximab plus concomitant boost radiotherapy in Japanese patients with locally advanced squamous cell carcinoma of the head and neck. Jpn J Clin Oncol 43:476–482
Acevedo-Henao CM, Valette G, Miglierini P et al (2012) Radiotherapy combined with cetuximab for locally advanced head and neck cancer: results and toxicity. Cancer Radiother 16:601–603
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethical Standards
The study was performed in accordance with the ethical standards as laid down in principles of Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rawat, S., Jain, R.K. & Verma, C. Cetuximab Concurrent with Radiotherapy in Unresectable, Locally Advanced Squamous Cell Carcinoma of Head and Neck: Real-World Evidence from a Tertiary Care Hospital. Indian J Otolaryngol Head Neck Surg 74 (Suppl 2), 1857–1863 (2022). https://doi.org/10.1007/s12070-020-01877-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-020-01877-y