Abstract
Mutations of p53 gene is one of the most common events in human cancers including oral squamous cell carcinoma (OSCC). However, its role in carcinogenesis and association with regard to prognosis is still under investigation and unclear. The aim was to study the expression of p53 in patients of OSCC and correlation with clinical presentation and prognosis. In this retrospective observational pilot study, we examined expression of p53 in 50 histologically diagnosed cases of OSCC and correlated it with initial clinical presentation and clinical events in follow up period. p53 expression was significantly negative (94%) in patients with history of only oral tobacco consumption while cases with additional history of smoking or alcohol were positive (p = 0.0001 and 0.011). On the other hand, aggressive course of the disease was found to be significant with p53 positivity in the form of lymph nodal extension (13 out of 17 cases) (p = 0.011) and recurrence (6 out of 10 cases) (p = 0.024). p53 was overexpressed (positive) in predisposing factors like smoking and alcohol but not in OSCC associated with chewing tobacco. p53 overexpression is also associated with advanced TNM stage. To the best of our knowledge, this is the first report where association of p53 overexpression and oral tobacco consumption associated OSCC was not observed and we recommend that carcinogenic events in chewing tobacco induced OSCC should be studied separately for its unique set of mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral cancer is the 6th most common cancer worldwide accounting for 4% of all cancers. However; it is a much bigger health problem in south East Asian countries where it may comprise upto 50% of all cancer in certain geographic areas [1]. In India, tobacco in both forms, chewable as well as nonchewable is responsible for majority of oral squamous cell carcinoma (OSCC). According to oral cancer statistics, oral cancer had a maximum age adjusted incidence rates (64.8%) in central India [2]. According to Global Adult Tobacco Survey, 34.6% Indian adults consume tobacco out of which 14% smoke tobacco while 25.9% consume smokeless tobacco [3]. The development of OSCC is a multistep process requiring the accumulation of multiple genetic alterations, influenced by a patient’s genetic predisposition as well as by environmental influences including tobacco, alcohol, chronic inflammation and viral infection [4]. In Indian subcontinent, tobacco abuse is mainly in the form of chewing tobacco which is seldom studied separately for its own unique characteristics.
Recent advances in the field of tumour-suppressor genes and oncogenes have provided an important tool for studying the genetic changes occurring at different stages of carcinogenesis, including transition from premalignancy to malignancy [5]. Mutations in p53 gene is one of the most common and early events in human cancers, including OSCC [6]. Ghanghoria S et al. [7] suggested that mutation of p53 gene leads to the protein product which is non-functional though expressed in tumour cells and can be detected by immunohistochemistry (IHC).
The goal of the present study was to investigate significance of p53 tumour marker in patients of OSCC in study area having a very high incidence of this malignancy.
Materials and Methods
Case Selection
It was a retrospective observational pilot study. After taking approval from Institutional ethical committee, 50 known consecutive patients of histologically diagnosed OSCC were selected as study cases. These were diagnosed minimum 12 months back from the initiating point of the study and were then followed up for next 2 years with their consent. The details of cases were retrieved from hospital records along with paraffin blocks and all available slides. After case selection, patients were contacted on their follow up visits, informed consent was taken and they were interviewed for the course of their disease. H&E stained slides were screened to obtain the best section for IHC. Immunohistochemical stain for p53 was performed by standard technique [8]. Cases that didn’t give consent or were lost to follow up or patients who died soon after initial diagnosis were not included in the study. Patient confidentiality was maintained during all research procedures.
Immunohistochemistry
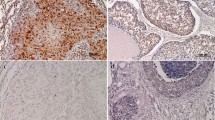
Cases along with appropriate positive and negative controls were stained with p53 (6 mL ready to use mouse monoclonal IgG2a primary antibody Anti p53 protein, BioGenex, USA). The intensity of immunohistochemical staining [9] was graded based on subjective evaluation of brown colour as: negative (−, no colour), mild (+ , light brown colour), moderate (++ , dark brown colour) or intense (+++ , very dark brown colour). Cases with (−) and (+) were considered as negative while (++) and (+++) were considered as positive. Only nuclear staining of epithelial cells were observed. The pattern of expression was also analysed semi quantitatively by counting the number of positive cells per 100 tumour cells and was recorded as percentage. The percentage of positive cells was scored as: 0 = 0–5%; 2 = 6–25%; 4 = 26–60%; 6 = 61–99%. The area with maximum number of positive cells was considered in each section. Cases with score 0 and 1 were considered as negative while 3 and 4 were considered as positive (Figs. 1 and 2). Known positive immunostaining slides were used as positive controls.
OSCC showing weak nuclear expression of Wildp53 in the dysplastic epithelium on left upper corner but not in the pushing border of invasive carcinoma in lower field (× 400, IHC); the case was considered as 10% × 0 in terms of percentage of cells x intensity of staining in a case of patient with history of chewing tobacco only
Patient Follow Up
All outdoor and indoor visits of study cases to the hospital after primary management were recorded using (1) records of ENT Department (2) of Radio chemo-oncology department (3) District cancer registry working from Department of Pathology and (4) our computerized hospital information system (HIS). During follow up period, the patients were evaluated for any adverse outcome in particular.
Statistical Analysis
Statistical analysis was done by using descriptive and inferential statistics using chi square test and fisher’s exact test. Software used was SPSS17.0 and graph pad PRISM 6.0 version and p < 0.05 was considered as level of significance.
Results
More than two third (72%) of cases were males in the present study with highest number of OSCC was present in 41–50 years of age group. On TNM staging, maximum (42%) cases were in TNM stage IV, 26% each in stage II and Stage III and only 6% in stage I. All T4 primary tumours (24%) presented in stage IV with N2 LN positivity in all cases. Ten out of 13, stage III disease cases also had N1 LN positivity.
Eighteen patients had history of tobacco chewing only; while 13 were smokers also, in addition to habit of tobacco chewing. 14 patients had history of combined tobacco and alcohol abuse. 5 patients of OSCC had no history of tobacco abuse. Overall 90% had history of tobacco abuse. We found that patients with history of chewing tobacco only were not associated with p53 over expression as percentage of positive cells or intensity of staining (4 & 1 out of 18) while there was over expression (both percentage of positive cells and intensity of smoking) with smoking and alcohol abuse (12 & 11 out of 13), also (8 & 6 out of 14) with highly significant p values in all categories (Table 1).
The adverse outcome in our study was defined as recurrence of cancer at primary site, distant metastasis or death after completion of primary treatment. Non-compliance of long, painful and costly treatment of cancer in our resource poor clientele was a hindering factor to study aggressiveness of primary tumour. So, for adverse outcomes, we followed only 27 cases who completed the treatment and out of these 9 cases presented with complications during 4 year follow up. 60% (n = 6/10) of p53 positive cases had aggressive course while only 3 out of 17 negative cases had complications after completion of primary treatment. Statistically significance was seen for both percentage of cells showing staining as well as intensity of staining (p = 0.024 and p = 0.029) (Table 2).
Well differentiated OSCC was the largest group comprising of 76% of total cases (n = 38)., p53 mutation was highly present in significant number of cases presenting with advanced stage with lymph node positivity (13 out of 17); both in terms of percentage of positive cells as well as intensity of staining (p = 0.0002 and p = 0.011 respectively) (Table 3).
Discussion
TP53 gene is called as the “Guardian of the Genome”. It is usually in a standby mode and its activation occurs in response to a variety of exogenous and endogenous cellular stresses. Activation of the gene normally results in formation of the p53 protein which has 393 amino acids and this protein ultimately causes either cell cycle arrest or apoptosis. In a normal cell, its activity is strictly regulated as it is a biologically active molecule. It is very rapidly degraded and hence difficult to detect by IHC [10, 11]. Due to mutation of this gene in cancer cells, the altered protein gets stabilized thus curtailing its degradation. Further, these mutations have loss of growth suppressor function as well as gain of function, together makes them more resistant to anti-cancer drugs and thus metastases and poor survival in the patients OSCC [10,11,12,13,14].
We followed our patients that were diagnosed at least 12 months back from initiating point of study for any adverse outcomes, to have minimum 3–4 years follow up period in each case. In our study, aggressive course of the disease was found to be associated significantly with p53 positivity in the form of lymph nodal extension even in WD OSCC (13 out of 17 cases) (p = 0.011) and recurrence (6 out of 10 cases) (p = 0.024). This shows that p53 expression was associated with more aggressive course of the disease. Carlos de Vincente J et al. [12] investigated OSCCs without initial neck node metastases and found that p53 expression was a significant prognostic factor and was associated with longer survival in patients with p53 negative tumours. de Olieveira et al. [11] in 2007 in a study of 107 cases of OSCC concluded that p53 overexpression is associated with a large number of metastasis (78.6%, p = 0.002) as well as poor outcome Negative p53 expression had a statistically significant better prognosis as compared to p53 positive cases, p = 0.046). Unal et al. [15] reported a significant correlation of immunoreactivity of p53 correlated with larger primary tumour size (p < 0.05), lymph node metastases (p < 0.05) and with pathological tumour stage (p < 0.05). Jayade BV et al. [16] in 2009 in a study of 90 resected specimen of stage IV SCC buccal mucosa found a significant correlation between p53 overexpression with histological grade and high cellular turn over. Both of these signify a poor prognosis for OSCC. Other similar studies indicating lymph node metastasis and poor survival rate leading to poor prognosis in patients includes studies done by Motta Rda R et al. [17], Nader S et al. [18] and Dave KV et al. [19].
One very interesting finding in our study was, however, absence of p53 mutation with a specific type of tobacco abuse in Indian subcontinent i.e. chewing tobacco. We could not find any previous study reporting this finding, especially in Central India. We found that these patients with tobacco abuse in chewing form comparatively had good prognosis and less number of adverse outcomes in 3–4 year of follow up period, which is most likely associated with absence of p53 mutation. This suggest that p53 mutation is not the predominant route of carcinogenesis with chewing tobacco, also have significantly less number of adverse outcomes in our study.
Brennan JA et al. [20] reported p53 mutations were found to be higher in smokers than non-smokers and even higher in smokers who also drink alcohol which was also observed in one study.
Conclusion
-
1.
p53 expression is associated with more aggressive course of disease.
-
2.
Tobacco abuse in chewing form is found to have better prognosis; may be because of absence of p53 mutation.
We feel that a direct comparison between these parameters will provide a clue to the exact role of p53 in carcinogenesis as well as it will help in understanding the prognosis of patients in future. This finding is significant in context of Indian subcontinent and should be explored in further studies.
References
Kaur J, Srivastava A, Ralhan R (1994) Overexpression of p53 protein in betel- and tobacco related human oral dysplasia and squamous-cell carcinoma in India. Int J cancer 58(3):340–345
National Centre for Disease informatics and Research. National Cancer Registry Programme: Five years Report of Population Based Cancer Registry, Wardha District: 2010–14, Maharashtra, India. Indian Council of Medical Research; July 2016:14.
Choi S, Myers JN (2008) Molecular pathogenesis of oral squamous cell carcinoma: Implications for therapy. J Dent Res 87(1):14–32
Prives C, Hall PA (1999) The p53 pathway. J Pathol 187(1):112
Abbas NF, El-Sharkawy SL, Abbas EA, El-Shaer MAM (2007) Immunohistochemical study of p53 and angiogenesis in benign and preneoplastic oral lesions and oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology 103(3):385–390
Ghanghoria S, Ghanghoria A, Shukla A (2015) p53 Expression in Oral cancer: a study of 50 cases. J Pathol Nepal 5:747–751
SSTM IHC Setection systems. Instructions manual. Biogenex. Doc No. 932-IHCMANE. Rev. no.: E, release date: 25th May 2011, p.12–6.
Humayun S, Prasad VR (2018) Expression of p53 protein and Ki-67 in oral premalignant lesions and oral squamous cell carcinomas: an immunohistochemical study. J Int Soc Prev Community Dent 8(6):513–522
Gasco M, Crook T (2003) The p53 network in head and neck cancer. Oral Oncol 39(3):222–231
Guimaraes DP, Hainaut P (2002) TP53: a key gene in human cancer. Biochimie 84(1):83–93
Carlos de Vicente J, Junquera Gutierrez LM, Zapatero AH, Fresno Forcelledo MF, Hernandez-Vallejo G, Lopez Arranz JS (2004) Prognostic significance of p53 expression in oral squamous cell carcinoma without neck node metastases. Head Neck 26(1):22–30
Cruz I, Snijders PJ, Van Houten V, Vosjan M, Van der Waal I, Meijer CJ (2002) Specific p53 immunostaining patterns are associated with smoking habits in patients with oral squamous cell carcinomas. J Clin Pathol 55(11):834–840
Yamazaki Y, Chiba I, Hirai A, Notani K, Kashiwazaki H, Tei K, Totsuka Y, Iizuka T, Kohgo T, Fukuda H (2003) Radioresistance in oral squamous cell carcinoma with p53 DNA contact mutation. Am J Clin Oncol 26(5):e124–129
de Oliveira LR, Ribeiro-Silva A, Zucoloto S (2007) Prognostic impact of p53 and p63 immunoexpression in oral squamous cell carcinoma. J Oral Pathol Med 36(4):191–197
Unal OF, Ayhan A, Hosal AS (1999) Prognostic value of p53 expression and histopathological parameters in squamous cell carcinoma of oral tongue. J Laryngol Otol 113:446–450
Jayade BV, Bhat K, Patil BR, Nayak R, Sant A (2009) Histological significance of p53 gene expression in squamous cell carcinoma of the buccal mucosa. J Maxillofac Oral Surg 8(3):205–210. https://doi.org/10.1007/s12663-009-0051-6Epub 2009 Nov 21
Motta Rda R, Zettler CG, Cambruzzi E, Jotz GP, Berni RB (2009) Ki-67 and p53 correlation prognostic value in squamous cell carcinomas of the oral cavity and tongue. Braz J Otorhinolaryngol 75(4):544–549
Nader S, Soheila N, Nastaran R, Maryam S (2015) The diagnostic value of the P53 tumor marker as a prognostic factor in patients with squamous cell carcinoma of the larynx. Biomed Pharmacol J 8:9–14 March Spl Edition
Dave KV, Chalishazar M, Dave VR, Panja P, Singh M, Modi TG (2016) Immunohistochemical expression of p53 and its clinicopathological correlation with modified Anneroth's histological grading system. J Oral Maxillofac Pathol 20(1):29–35. https://doi.org/10.4103/0973-029X.180922
Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, Couch MJ, Forastiere AA, Sidransky D (1995) Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med 332(11):712–717
Acknowledgements
We would like to acknowledge Kasturba Health Society, Sevagram, Wardha, Maharashtra, India for partial funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest for this study.
Research Involving Human Participants and/or Animals
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deshmukh, A.V., Gupta, A., Chaudhari, A.G. et al. Correlation of p53 expression with Clinical Presentation and Prognosis of Oral Squamous Cell Carcinoma Patients: A Pilot Study. Indian J Otolaryngol Head Neck Surg 74 (Suppl 2), 1836–1840 (2022). https://doi.org/10.1007/s12070-020-01859-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-020-01859-0