Abstract
Cochlear implantation (CI) is used for rehabilitation of children with bilateral severe to profound sensorineural hearing loss. Recently, treatment of such children has been influenced by diagnostic technological advances. Infants and toddlers are now increasingly included for CI. The primary aim of this study was to determine the effects of ‘age at CI’ on CI outcome. The primary aim of this study was to determine the effects of ‘age at CI’ on CI outcome. In this prospective study at a tertiary care centre, we evaluated 50 cochlear implanted children from October 2011 to March 2013. The case group consists of 15 (30%) children who underwent CI at more than 5 years of age and control group consisted of 35 (70%) children who underwent CI at less than or equal to 5 years age. All patients received auditory and speech rehabilitation and we evaluated their auditory perception outcomes 1 year post CI, the children were assessed by categories of auditory performance (CAP) and meaningful auditory integration scale (MAIS) tests. There were significantly improved mean auditory perception outcomes (increase of 12.29% in CAP, and 14.05% in MAIS scores) at 1 year post CI in CI recipients of age group ‘5 years or less’ in comparison to those who underwent CI at ‘more than 5 years of age’. However, children of ‘more than 5 years’ age at CI, mean CAP and MAIS scores were still more than 80% of maximum achievable CAP and MAIS scores. In this study, CI recipient children who were implanted at less than or equal to 5 years of age were found to have significantly improved auditory perception outcome at 1 year post CI. Hence, it appears preferable to provide CI early. However, even in children who underwent CI at more than 5 years of age, there was substantial improvement in auditory perception outcomes and CI was still helpful in these children. Hence, knowledge of ‘age at CI’ can provide reasonable help in predicting the auditory perception outcome and optimal counselling of families of CI candidates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over a period of more than 3 decades, cochlear implantation (CI) is firmly established as safe and effective treatment of choice for children with bilateral severe to profound sensorineural hearing loss (SNHL) who derive minimal or no benefit from conventional hearing aid use. The primary aim of CI is to enable a child to improve auditory perception and hence spoken language. The development of functional spoken language would be considered by most clinicians, teachers and parents to be a major long-term aim of CI. It is clear however that a properly functioning cochlear implant does not guarantee this outcome. The detection and discrimination of sound does not ensure that a child will be able to adequately assemble the complex stream of auditory information in speech into a meaningful language [1].
On the other hand, it is reasonable to assume that the auditory perception and comprehension of speech is an important ingredient in language development hence measurement of auditory perception will have some relationship with speech and language abilities. The measurement of auditory perception also provides direct evidence of the assistance provided by the CI [2]. Hence one of the most direct way to evaluate the benefit from a CI is by demonstrating improvement in auditory perception.
The variability in auditory perception outcomes for CI recipient children has been a subject of various studies. In the past 25 years, data on the postoperative auditory perception outcomes following CI have identified multiple variables that can affect post CI performance. On a cellular level ‘age at CI’ is believed to affect the survival and function of spiral ganglion cell which in turn influences the post CI performance. Despite extensive research examining post CI auditory outcomes, the substantial variation in postoperative performance still remains incompletely understood. Hence predictions of post CI auditory benefit should be individualized to specific patient, surgical or CI device related variable. Detailed knowledge of such individual variables not only improves clinician’s predictive accuracy but may also reveal factors that can be manipulated to achieve an optimal performance.
In this study, we explore a demographic variable of ‘age at intervention i.e. CI (in years)’ and its effect on auditory perception outcomes in CI recipient children at an Indian tertiary care centre. This may help to explain to a certain extent the variability in language outcomes with ‘age at CI’ and also determine the ‘age group’ for CI at which children are more likely to have improved auditory/language outcomes.
There are multiple studies on various patient, surgical or CI device related factors affecting auditory perception outcomes after CI [3, 4]. However adequate Indian data is not available on effects of ‘age at CI (in years)’ on auditory perception outcomes in CI recipient children. This study may provide useful information for counselling Indian families that are considering CI for their child regarding appropriate age for CI and may also help in predicting the auditory perception/language outcomes for individual CI candidates prior to CI surgery.
Materials and Methods
Study Design and Participants
This prospective study was performed on total 50 children with bilateral severe to profound sensorineural hearing loss (SNHL) that underwent CI at an Indian tertiary care centre between October 2011 and March 2013. The children who were selected included these criteria: (1) bilateral severe to profound SNHL, (2) onset of hearing loss before 6 months of age, (3) children undertaking their first CI, (4) the use of amplification and/or intervention program emphasizing spoken language, (5) the maximum age was 10 years old, and (6) undertaking rehabilitation at tertiary care hospital after their CI. We enrolled all those children who had undergone CI based on aforementioned selection criteria and then divided them into two groups (i.e. case and control groups) based on ‘age at CI’. Every patient was assessed by a clinical psychologist, a paediatrician, an audiologist and speech/language therapist.
Intervention
CI is usually performed on children with bilateral severe to profound SNHL. Hearing skill means ability to understand voices which are assessed by scores such as categories of auditory performance (CAP) and meaningful auditory integration scale (MAIS) (Tables 1, 2) [5, 6]. The bilateral severe to profound SNHL was confirmed by speech or pure tone audiometry (unaided/aided hearing thresholds), auditory brainstem response with click and tone burst methods, otoacoustic emissions, auditory steady state responses and impedance audiometry. All children had the no/minimal experience of speech detection/perception from properly fitted high gain hearing aids. All patients received audiological, speech perception, language skills, neurological, and psychological assessment immediately before CI. Imaging study consisted of HRCT temporal bones and/or MRI inner ear, internal acoustic meatus and brain for finding the central nervous system and temporal bone abnormalities. Age at CI (in years) was noted. Surgical approach consisted of cortical mastoidectomy under general anaesthesia. By posterior tympanotomy, the middle ear space was entered. Then, the bone at round window niche was drilled and round window membrane was perforated to complete cochleostomy. Finally, CI was secured and electrode array was inserted into the cochlea. After insertion, correct placement of electrode array was evaluated intraoperatively by neural response telemetry (NRT)/neural response imaging (NRI) and postoperatively by X-ray mastoid (modified Stenver’s view).
Outcome Assessments
Auditory skills defined as the ability of sound understanding which is assessed on CAP and MAIS criteria. All children were examined by questionnaires such as CAP and MAIS to measure the auditory perception development in children [5, 6]. Auditory perception in children using cochlear implants was studied in relation to the patient variable of ‘age at CI’ (whether 5 years or less/more than 5 years). Children with age at CI being ‘5 years or less’ were placed in a case group and ‘more than 5 years’ in a control group. CAP and MAIS scores were calculated before CI and also 1 year after CI.
Scientific and Ethical Considerations
All procedures were approved by children’s parents. This study was approved by hospital ethics committee.
Statistical Analysis
Relationship of the variable ‘age at CI (in years)’ to auditory perception outcome (mean CAP and MAIS scores) were analysed 1 year post implant using Chi square test, paired test and independent t test.
Results
Total 50 children had undergone CI over a period of 18 months. Out of them, only 15 (30%) underwent CI at ‘more than 5 years’ of age, while 35 children (70%) underwent CI at the age of ‘5 years or less’. Among children with age at CI being more than 5 years, 08 (53.3%) were male and 07 (46.7%) were female. The mean age of all participant children was 5.06 years. The mean age of case group with age at CI being ‘5 years or less’ was 4.4 years and mean age of control group with age at CI being ‘more than 5 years’ was 6.6 years. Mean CAP and MAIS scores were calculated for both case and control groups at 1 year after CI (Tables 3, 4, 5, 6). Maximum achievable CAP score (CAPmax) was 7 and MAIS score (MAISmax) was 40.
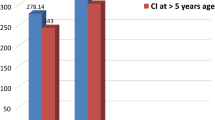
In this study, for CI recipient children with ‘age at CI’ being ‘5 years or less’, the mean CAP score was 6.49 and mean MAIS score was 37.26 at 1 year after CI. However, for children whose ‘age at CI’ was ‘more than 5 years’, the mean CAP score was 5.67 and mean MAIS score was 32.67 at 1 year after CI (Table 7, Fig. 1). Hence, there were significantly improved mean auditory perception outcomes (increase of 12.29% in mean CAP, and 14.05% in mean MAIS scores) in CI recipients at 1 year after CI when they were implanted at the age group of ‘5 years or less’ as compared to those who were implanted at ‘more than 5 years’ of age.
Additionally, in this study we have found that in children aged ‘more than 5 years’ at CI, mean CAP score was 5.67 (81% of CAPmax) and mean MAIS score was 32.67 (81.7% of MAISmax) at 1 year after CI. Although this was lesser when compared to the case group with ‘5 years or less’ of age at CI having mean CAP score of 6.49 (92.7% of CAPmax) and mean MAIS score was 37.26 (93.2% of MAISmax) but it still showed substantially improved mean auditory perception outcomes at 1 year after CI (Table 7).
Discussion
This study was performed to evaluate the effect of age at CI being ‘5 years or less’ and ‘more than 5 years’ on auditory perception outcomes 1 year after CI in children with bilateral severe to profound SNHL. The effects of bilateral severe to profound hearing loss are widely known to be serious, especially in relation to understanding and using spoken language.
The primary auditory cortex (PAC) does not develop entirely normally in the absence of sensory input. Therefore, CI appears to allow the PAC to achieve an experience dependent maturation, albeit not as precisely as it would have occurred in the absence of SNHL. There is also a positive correlation between speech perception and low resting activity in PAC prior to CI for the prelingual deaf. This relationship suggests that although PAC and other higher order auditory centres are capable of plastic change as evident in the continued improvement in the auditory performance with increasing CI experience. Perhaps the best clinical outcomes for cochlear implant patients may in fact occur with the more immature auditory cortex [7].
As CI becomes a more common and trusted option for bilateral severe to profoundly deaf children and their families, a great deal of research has been dedicated to the effects of early implantation. ‘Age at CI’ relates to the survival, physiology and function of spiral ganglion cells. Positive effects of CI on spiral ganglion cells have been well shown in animal models. However, a critical period for the development of human auditory pathway has not been definitively established, although research in this area is in progress. Effects of deprivation and plasticity on post CI performance can be interpreted to a certain extent via the variable of ‘age at CI’ [8].
Extensive newborn hearing screening has led to an increase in early diagnosis and larger opportunities for early CI, including children younger than 1 year. CI in children younger than 1 year of age has shown both short-term and long-term safety and efficacy. Using the MAIS score to evaluate auditory perception, Waltzman and Roland [9] and Roland Jr. et al. [10] suggested that CI before 1 year of age may allow deaf children to reach their full hearing potential, sometimes paralleling the normal-hearing peers. Colletti et al. [11] used CAP scores in their study of 10 children younger than 1 year. They found that auditory outcomes in these children younger than 1 year age exceeded those of children who underwent CI later. Niparko et al. [12] showed improved speech and language outcomes in children who underwent CI before 18 months of age, mostly paralleling the performance of normal-hearing peers. Connor et al. [13] and Miyamoto et al. [14] found improved auditory perception and language skills in children receiving CI at less than 2 years of age when compared to children older than 2 years. Tajudeen et al. [15] found a benefit of early CI when comparing children of the same age, but not when comparing children at the same time after CI. For this reason, they postulated that the sensitive period for word identification likely extends to at least 3 years of age. In a study of children implanted between 9 and 48 months, Hammes et al. [16] found that being implanted as early as possible was a significant predictor of more positive outcomes with the implant. On average, the younger the child was implanted, the higher the likelihood for that child to develop auditory and language skills to allow that child to rely on spoken language as a sole means of communication. As age of implantation increased, the children began to lag further behind in auditory/language performance with their age matched peers with comparable duration of CI usage [16]. Even in adolescent CI recipients, studies suggest that CI performance is affected by ‘age at CI’. A study of 45 prelingually deafened adolescents with a mean ‘age at CI’ of 13.5 years (range 11–18 years) found ‘age at CI’ to affect auditory perception outcomes. However, after CI all patients showed significant improvement from preoperative scores [17].
Although evidence regarding critical periods for auditory and linguistic development continues to emerge, the factor of ‘age at CI’ seems to have a clear impact on CI performance in children. As technology has evolved, CI outcomes for the congenitally deaf later-implanted group, has also shown substantial improvement [18].
The arguments that younger children should perform better and the available research evidence have continuously reduced the average age at implantation in most CI programmes [19]. However, there are risks in operating children younger than 12 months of age. Young children have an underdeveloped mastoid tip, a thin skull, a thin skin and a higher risk of complications after anaesthesia. As per one study, comparing children with CI, who received their implant at 6 months of age, with children implanted at the age of 12 months, it was found that the advantages from implanting children at 6 months of age were rather small and therefore the risks of the treatment should be taken into consideration [8]. Since the decision to surgically implant the device in a very young child is quite difficult on the part of the parent and the clinician, researchers have attempted to discover whether or not early implantation provides advantages in outcome measures post-implantation as compared to later implanted children. If such an advantage exists, it is also important to understand how early the CI should be performed to obtain more positive results, without incurring unnecessary surgical risks. Nonetheless, it appears preferable to provide a cochlear implant at an early age.
In this study, we have also found significantly improved mean auditory perception outcomes (increase of 12.29% in CAP and 14.05% in MAIS scores) in CI recipients when they were implanted at age group of ‘5 years or less’ when compared to those who were implanted at ‘more than 5 years’ of age [P value < 0.05 (significant) for both mean CAP and mean MAIS scores] [20]. Additionally, in this study children with control age group of ‘more than 5 years’ at CI, mean CAP and mean MAIS scores were more than 80% of CAPmax and MAISmax respectively at 1 year after CI. This is albeit less than the case age group of ‘5 years or less’ at CI, with mean CAP and mean MAIS scores of more than 90% of CAPmax and MAISmax respectively at 1 year after CI. Hence it is safe to conclude that even in children aged more than 5 years at CI surgery, there is substantial improvement in auditory perception outcomes at 1 year after CI if we assume their mean CAP and MAIS scores before the CI surgery to be near ‘0’.
However, as per Government of India guidelines for CI under ‘Assistance to disabled persons for purchase/fitting of aids and appliances’ (ADIP) scheme provide general age limit of 1–5 years for prelingually deaf children with bilateral severe to profound SNHL. Additionally, as per the ‘Central government health scheme’ (CGHS) rules, in prelingually deaf children aged between 1 and 5 years with bilateral severe to profound SNHL, there is 100% reimbursement of the total cost of cochlear implant. However, for children with more than 5 years of age, the reimbursement value is only 50–80% of the total cost of implant [21, 22]. This gives an impression that speech perception outcomes of CI in children with age of more than 5 years would generally be suboptimal. However, in this study we have clearly found substantial benefit in auditory perception outcomes at 1 year after CI even in children aged ‘more than 5 years’ at CI. Hence CI programmes may also be supportive of the age group of ‘more than 5 years’ at CI, especially in developing countries where there are financial and medical infrastructure related constraints for families of children with bilateral severe to profound SNHL.
Conclusion
There are multiple studies on various patient, surgical or CI device related factors affecting auditory perception outcomes after CI [3, 4]. However adequate Indian data is not available on effects of ‘age at CI (in years)’ on auditory perception outcomes in CI recipient children especially ‘more than 5 years’ age group. This study may provide useful information for counselling Indian families that are considering CI for their child regarding appropriate age for CI or expected outcomes in CI at ‘more than 5 years’ of age. Additionally, this study may also help in predicting the auditory perception/language outcomes for individual CI candidates prior to CI surgery.
Hence it is safe to conclude that in Indian cochlear implant scenario, knowledge of the individual factor of ‘age at CI’ can provide reasonable help in predicting the auditory perception and hence spoken language outcomes for individual implant candidates prior to the CI surgery. However, the accuracy of such predictions is limited and they should only be used as a guide towards predicting auditory perception outcomes.
References
Miyamoto RT, Osberger MJ, Todd SL, Robbins AM, Stroer BS, Zimmerman-Phillips SZ et al (1994) Variables affecting implant performance in children. Laryngoscope 104:1120–1124
Dowell RC, Cowan RSC (1997) Evaluation of benefit: infants and children. In: Clark GM (ed) CI for infants and Children—advances. Singular Publishing Group, San Diego, pp 205–222
Osberger M (1998) Speech recognition performance of older children with cochlear implants. Am J Otol 19:152–157
Tye-Murray N, Spencer L, Woodworth GG (1995) Acquisition of speech by children who have prolonged cochlear implant experience. J Speech Hear Res 38:327–337
Archbold S, Lutman ME, Marshal DH (1995) Category of auditory performance. Ann Otol Rhinol Laryngol 166(Suppl. 1):312–314
Zimmerman-Phillips S, Robbins AM, Osberger MJ (2001) Infant–toddler meaningful auditory integration score. Calif. Advanced Bionics Corp, Sylmar
Blamey P, Arndt P, Bergeron F (1996) Factors affecting auditory performance of postlingually deaf adults using cochlear implants. Audiol Neurootol 1:293–306
Holt RF, Svirsky MA (2008) An exploratory look at pediatric cochlear implantation: is earliest always best? Ear Hear 29(4):492–511
Waltzman SB, Roland JT Jr. (2005) Cochlear implantation in children younger than 12 months. Pediatrics 116(4):e487–e493
Roland JT Jr., Cosetti M, Wang KH et al (2009) Cochlear implantation in the very young child: long-term safety and efficacy. Laryngoscope 119(11):2205–2210
Colletti V, Carner M, Miorelli V et al (2005) Cochlear implantation at under 12 months: report on 10 patients. Laryngoscope 115(3):445–449
Niparko JK, Tobey EA, Thal DJ et al (2010) Spoken language development in children following cochlear implantation. JAMA 303(15):1498–1506
Connor CM, Craig HK, Raudenbush SW et al (2006) The age at which young deaf children receive cochlear implants and their vocabulary and speech-production growth: is there an added value for early implantation? Ear Hear 27(6):628–644
Miyamoto RT, Hay-McCutcheon MJ, Kirk KI et al (2008) Language skills of profoundly deaf children who received cochlear implants under 12 months of age: a preliminary study. Acta Otolaryngol 128(4):373–377
Tajudeen BA, Waltzman SB, Jethanamest D et al (2010) Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Otol Neurotol 31(8):1254–1260
Hammes DM, Novak MA, Rotz LA et al (2002) Early identification and cochlear implantation: critical factors for spoken language development. Ann Otol Rhinol Laryngol Suppl 189:74–78
Arisi E, Forti S, Pagani D et al (2010) Cochlear implantation in adolescents with prelinguistic deafness. Otolaryngol Head Neck Surg 142(6):804–808
Waltzman S, Roland JT, Cohen N (2002) Delayed implantation in congenitally deaf children and adults. Otol Neurotol. 23:333–340
Sarant J, Blamey PJ, Dowell RC, Gibson WPR (2001) Variation in auditory perception scores amongst children with cochlear implant. Ear Hear 22(1):18–28
Fisher RA (1950) Statistical methods for research workers. Oliver and Boyd, London
Government of India. Ministry of social justice and empowerment (2015). Corrigendum—guidelines approved for hearing impaired for financial assistance under revised ADIP scheme-modifications regarding [Report No. 4-2 (8)/2014/DD-I]. Department of Empowerment of Persons with Disabilities, New Delhi
Government of India. Ministry of health and family welfare (2009). Office memorandum—reimbursement of the cost of cochlear implant to beneficiaries under CGHS/Central Services (Medical Attendance) Rules, 1944 (File No. 6-469/2003-CGHS/R&H). Department of Health and Family Welfare, New Delhi
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Dr. Vishal Gaurav: Concept and design of study, acquisition of data/analysis and interpretation of data. Dr. Shalabh Sharma: Manuscript review and final approval of the version to be published. Dr. Satinder Singh: Manuscript review.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gaurav, V., Sharma, S. & Singh, S. Effects of Age at Cochlear Implantation on Auditory Outcomes in Cochlear Implant Recipient Children. Indian J Otolaryngol Head Neck Surg 72, 79–85 (2020). https://doi.org/10.1007/s12070-019-01753-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-019-01753-4