Abstract
Respiratory epithelial adenomatoid hamartoma (REAH) is a distinct non-neoplastic entity originating from anterior olfactory cleft in the nasal cavity, often going unnoticed. Clinically, REAH presents as unilateral or bilateral nasal polyps. Our aim is to expand the understanding of bilateral REAH associated with nasal polyposis with respect to clinical, radiological and histopathological features for better clinical outcomes. Our analysis includes patients presenting as bilateral nasal polyps, whose CT-PNS showed opacity in olfactory clefts. During endoscopic sinus surgery, the lesions in the olfactory cleft (medial-to-middle turbinate) were identified and the specimens from olfactory cleft and ethmoid sinus cavity were subjected separately to histopathological analysis. Six patients (average age 50 years, 83% male) of bilateral REAH with nasal obstruction of > 3 years were analysed. On nasal endoscopy, the polypoid masses in the olfactory cleft and in the ethmoids did not show any gross differences. However, polypoidal masses from the olfactory cleft bled more during biopsy and excision. Histopathological study of these masses revealed the closely arranged round to oval glands (with few dilated glands) lined by ciliated columnar epithelium in mildly edematous stroma, confirming the presence of REAH. REAH is an often overlooked lesion in the nasal cavity, arising from olfactory cleft. The presence of nasal polyposis obscures this lesion, resulting in under diagnosis. The prompt identification with high index of suspicion by the otorhinolaryngologists helps in accurate histopathological diagnosis thereby improving clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “hamartoma” (in Greek ‘hamartia’ meaning “to fail or to err” and ‘-oma’ denoting “a tumor or neoplasm”) was first used and described by a German Pathologist, Albrecht in 1904. Hamartoma can be defined as focal malformation that resembles a benign neoplasm, comprised of disorganized cytologically normal mature cells and tissues of its origin [1]. Hamartomas can occur at any location in the body mostly originating from skin or subcutaneous tissue, kidney, lung, liver, spleen and gastrointestinal tract [2,3,4], but involvement of the head and neck is exceedingly unusual. Hence, these hamartomas are rarely encountered by otolaryngologists. In the head and neck, the common sites of occurrence of hamartomas are soft tissue cheek, soft tissue neck, maxilla, gum margin and tongue [2]. Of special interest among hamartomas of the head and neck are those which arise in nose and PNS with predominance of cells of respiratory epithelial origin. Respiratory epithelial adenomatoid hamartoma (REAH) is a prominent glandular proliferation lined by ciliated respiratory epithelium arising from the surface epithelium, first described in 1995 by Wenig and Heffner [5]. Ten years later, REAH was included in the World Health Organization (WHO) Classification of Head and Neck Tumors in 2005 [6]. In nose and PNS, the most common site of REAH is the posterior part of the nasal septum [6, 7]. Other common sites include the nasopharynx, frontal, maxillary and ethmoid sinuses, mostly being unilateral [5, 8,9,10,11,12,13,14,15,16]. However, REAH arising in the anterior olfactory cleft is exceedingly rare and unique [17,18,19]. This study aims to expand the understanding of bilateral REAH originating from olfactory clefts and associated with nasal polyposis with respect to clinical, radiological and histopathological features for better clinical outcomes.
Materials and Methods
A total of 60 cases of bilateral nasal polyps were treated during the period of 12 months from October 2017 to September 2018 in the Department of ENT, SDM College of Medical Sciences & Hospital, Dharwad. Anterior rhinoscopy and diagnostic nasal endoscopic examination (4 mm, 0°, Karl Storz Se & Co. Germany) was performed in the out-patient department to confirm and to stage the nasal polyps according to the Lildholdt staging system. A detailed history, complete ENT and general physical examination, and routine hematological investigations were done. These patients were subjected to computed tomography-paranasal sinuses (CT-PNS) 1.0 mm axial cuts with coronal and sagittal reconstruction. Normal CT-PNS does not show any opacity in the olfactory clefts (Fig. 1a). Most cases of chronic rhinosinusitis (CRS) with polyps do not show opacities in the olfactory clefts (Fig. 1b). Excluding all these cases, only six cases showing soft tissue opacity in olfactory clefts (medial-to-middle turbinate) in the CT-PNS were recruited for the study (Fig. 1c, d).
Computed tomography of paranasal sinuses. a Normal CT-PNS with normal olfactory clefts. b Opacities in the ethmoids of chronic rhinosinusitis with normal olfactory clefts. c Opacities predominantly seen in the olfactory clefts. d REAH prolapsing anteriorly from the olfactory clefts. * Olfactory cleft
After obtaining written informed consent, patients were posted for endoscopic sinus surgery (ESS). None of these patients were administered preoperative systemic steroids. During endoscopic sinus surgery, the lesions in the olfactory cleft (medial-to-middle turbinate) were carefully identified and the specimens of polypoidal masses from olfactory cleft and ethmoid sinuses were taken separately for histopathological examination. Bilateral full house ESS was done. Microdebrider (Medtronic, USA) was used to debride the polyps. In comparison to ethmoidal polyps, the polypoidal masses medial-to-middle turbinate demonstrated more bleeding, which was controlled with bipolar coagulation. The polypoidal masses medial-to-middle turbinate were carefully debrided flush with olfactory cleft mucosa, taking care not to injure the cribriform plate, lateral lamella and the middle turbinate.
All the specimens were fixed in 10% formalin and the entire tissue was subjected to further processing. Sections of 5 μm thickness were stained with hematoxylin and eosin (H&E) stain. Microscopic evaluation was performed and REAH was diagnosed based on the WHO criteria—“glandular proliferation composed of widely spaced small to medium sized glands lined by multilayered ciliated respiratory epithelium often admixed with goblet cells. These glands arise in direct continuity with the surface epithelium and invaginate downwards into the submucosa” [6].
Results
Clinical Features
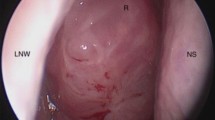
Out of 6 patients, 5 were men (83%), the age range of the patients was from 42 years to 62 years, with a median age of 50.5 years. There were multiple symptoms such as nasal obstruction, nasal stuffiness, hyposmia, facial pain, frontal headache and post-nasal drip. Nasal obstruction and hyposmia were the most common symptoms observed. These symptoms occurred over various periods time ranging from 1 to 8 years (Table 1). A detailed enquiry did not reveal correlation with any environmental or occupational etiological factors. Gross examination of the sinonasal tract revealed the presence of multiple polypoid or exophytic lesions with tan-white, yellow-pink to red-brown coloring, a glistening surface and a rubbery consistency. In these patients, the middle turbinates are thinned and pushed laterally by polypoidal masses occupying the olfactory cleft (Fig. 2a). The origin of polypoidal masses from the olfactory cleft was confirmed in all the cases by probing. The probe could not be passed between the masses and the olfactory cleft mucosa. In few cases, these masses were seen adherent to the adjacent surfaces of ipsilateral middle turbinate and nasal septum.
Computed tomography and endoscopy details. a Intraoperative endoscopic image of right nasal cavity showing the presence of REAH in the olfactory cleft (OC, olfactory cleft; MT, middle turbinate; NS, nasal septum; UP, uncinate process). b Coronal view of CT-PNS shows soft tissue opacities in bilateral olfactory clefts as well as in maxillary ethmoidal and frontal sinuses. c Lateral bowing of the middle turbinates widening the olfactory clefts. d Opacities only in the olfactory clefts with no opacities in the PNS (isolated REAH). * Olfactory cleft
Radiological Features
The CT-PNS of four patients showed homogenous soft tissue opacity in bilateral olfactory clefts along with similar opacities in the paranasal sinuses (Fig. 2b). In one case, not only was there opacity in the olfactory clefts but also clefts were widened significantly (the middle turbinates were pushed laterally with convexity towards lamina papyracea) (Fig. 2c). In another case, the opacities were restricted to the olfactory cleft and there were no opacities in the paranasal sinuses. Hence it was diagnosed as isolated REAH (Fig. 2d).
Histopathological Features
Histopathological studies revealed the presence of sinonasal mucosa lined by respiratory epithelium overlying small to medium sized glands embedded in fibrotic stroma (Fig. 3a). Additionally, the low-power microscopic examination showed respiratory mucosa overlying widely spaced medium sized glands lined by pseudostratified ciliated columnar epithelium admixed with few mucin secreting (goblet) cells, round to oval glands with stromal hyalinization (Fig. 3b, c). Furthermore, the high-power microscopy displayed glands invested in thickened eosinophilic basement membranes and surrounded by fibrotic stroma (Fig. 3d). Overall, the histopathological examination revealed the closely arranged round to oval glands (with few dilated glands) lined by ciliated columnar epithelium in mildly edematous stroma, confirming the presence of REAH.
Histopathological features of case 5. a Low-power photomicrograph displaying sinonasal mucosa lined by respiratory epithelium overlying small to medium sized glands embedded in fibrotic stroma. b Low-power microscopic examination showed respiratory mucosa overlying widely spaced medium sized glands lined by pseudostratified ciliated columnar epithelium admixed with few mucin secreting (goblet) cells. c Photo microscopy depicting proliferating round to oval glands with stromal hyalinization. d High-power photomicrograph displaying glands invested in thickened eosinophilic basement membranes and surrounded by fibrotic stroma
Discussion
Hamartomas are generally regarded as malformations composed of an excessive proliferation of a specific cellular component indigenous to a given tissue [6]. They are rare and distinct non-neoplastic lesions, composed of mature cells with no malignant potential and thus have self-limited growth [5, 8, 11]. In contrast to neoplasms, hamartomatous proliferations do not have the ability of continuous unimpeded growth. However, teratomas are neoplastic proliferations that are capable to grow continuously and composed of cells from all the three germ layers [5, 20, 21]. Hamartomas may arise in any site, most commonly seen in the lung, kidney, skin or subcutaneous tissue, liver, spleen and intestines [2,3,4]. However, the occurrence of hamartomas in the head and neck is exceedingly uncommon. The common sites of occurrence of hamartomas in the head and neck are soft tissue of cheek, soft tissue of neck, maxilla, gum margin, nose and tongue [2]. In the nose, hamartomas are most commonly located on the posterior aspect of the nasal septum [7, 22], followed by middle meatus, inferior turbinate, maxillary sinus, ethmoidal sinus and frontal sinus [8, 9, 11, 14, 15, 23]. These locations usually exhibit a unilateral solitary polypoidal mass, which are easily amenable for complete excision and biopsy. Histopathologically, in contrast to hamartomas of other locations, hamartomas of nose and PNS show predominance of respiratory epithelial cells. Hence, these hamartomas are named as “respiratory epithelial adenomatoid hamartoma” [5]. In the nose and PNS, the reports on hamartomas occurring in the olfactory clefts are very limited [17, 18, 24,25,26]. Also, there are no systematic studies on incidence of REAH among cases of nasal polyposis. In our study, we report six cases of REAH out of 60 cases presenting as bilateral nasal polyposis. These cases of REAH (hamartomas occurring in the olfactory clefts) are unique in the fact that they are bilateral and excluding one case, rest are associated with a bilateral nasal polyposis, which is an inflammatory pathology.
Radiologically, REAH is typically observed as a soft tissue opacity in the affected sinus with attachment to the posterior nasal septum, usually unilateral [5, 7]. However, this soft tissue opacity was indistinguishable from that of CRS as reported by Vira et al. [27]. In our study, similar radiological features were observed in all the cases. In addition, we observed homogenous soft tissue opacities in the olfactory clefts with no gap of translucency between the roof of olfactory clefts and the opacities. This confirmed the adherence of REAH to the olfactory mucosa in the cleft. The widening of olfactory cleft was bilateral and symmetric in our cases, similar observations were made in other studies. Additionally, the maximum widening at the mid-part of the vertical length of middle turbinate resulting in apparent bowing of middle turbinates with convexity facing laterally was observed in our cases.
The exact etiology of REAH is not known but bilateral olfactory cleft REAH are often associated with bilateral nasal polyps. As there are no known etiological factors associated with the development of REAH in our cases, the involvement of inflammatory process could be the main contributing factor [28]. REAH has been reported to have a genetic profile of increased fractional allelic loss of 31%. Since this is unusually high for a non-neoplastic entity like hamartoma, the possibility of whether it is a benign neoplasm is yet to be studied [6]. However, this hypothesis needs thorough investigation at a molecular level to prove the cause and effect relationship.
Histopathologically, REAH is characterised by adenomatoid proliferation of respiratory ciliated cells in the nasal cavity, sinuses and nasopharynx. The glandular component is derived from the surface nasopharyngeal epithelium and not from the seromucinous glands. Microscopically, at low magnification, the tissue is polypoidal and exhibits a benign proliferation of glands lined by ciliated respiratory epithelium. At places, the glandular lining is seen in direct continuity with the surface epithelium [29]. The stroma in REAH is edematous and contains mixed inflammatory infiltrate resembling that of an inflammatory polyp. A characteristic finding is hyalinization of stroma, with envelopment of glands by a thick eosinophilic basement membrane. Small reactive seromucinous glands can be seen amidst the proliferating glands [6]. REAH has to be differentiated from inflammatory polyps, and well differentiated adenocarcinoma. REAH does not exhibit cytological atypia, back to back cribriform pattern, and the desmoplastic stroma seen in adenocarcinoma [29, 30].
This study concludes that REAH is an often overlooked entity in the nasal cavity. REAH arising from olfactory cleft are often obscured by the ethmoidal polyps. Hence, we suggest the otorhinolaryngologists to have high index of suspicion of REAH whenever there is an opacity (in the CT-PNS) in the olfactory cleft associated with bilateral nasal polyposis. Therefore, the prompt identification of this masked entity would help in accurate histopathological diagnosis thereby improving clinical outcomes.
References
Terris MH, Billman GF, Pransky SM (1993) Nasal hamartoma: case report and review of the literature. Int J Pediatr Otorhinolaryngol 28:83–88
Jain RK (2003) Hamartoma of the head and neck. Indian J Otolaryngol Head Neck Surg 9:76–78. https://doi.org/10.1016/B978-0-7234-3261-6.50020-7
Liang J, O’Malley BW, Feldman M, Newman JG (2007) A case of respiratory epithelial adenomatoid hamartoma. Am J Otolaryngol 28:277–279. https://doi.org/10.1016/j.amjoto.2006.09.013
Ake Y, Kouame J, Moh E et al (2014) Skin hamartoma in children: diagnosis and therapeutic issues. J Pediatr Surg Case Rep 2:319–321. https://doi.org/10.1016/j.epsc.2014.06.007
Wenig BM, Heffner DK, Dennis Heffner CK et al (1995) Respiratory epithelial adenomatoid hamartomas of the sinonasal tract and nasopharynx: a clinicopathologic study of 31 cases. Ann Otol Rhinol Laryngol 104:639–645. https://doi.org/10.1177/000348949510400809
Wenig BM (2005) Respiratory epithelial adenomatoid hamartoma. In: Barnes L, Eveson J, Reichart P, Sidransky D (eds) WHO classification of tumors pathology and genetics of head and neck tumors. IARC Press, France, p 33
Park I-H, Lee H-MHC, Lee H-MHC et al (2013) Respiratory epithelial adenomatoid hamartoma originating from nasal septum. Clin Exp Otorhinolaryngol 6:45. https://doi.org/10.3342/ceo.2013.6.1.45
Himi Y, Yoshizaki T, Sato K, Furukawa M (2002) Respiratory epithelial adenomatoid hamartoma of the maxillary sinus. J Laryngol Otol 116:317–318. https://doi.org/10.1258/0022215021910672
Kessler HP, Unterman B (2004) Respiratory epithelial adenomatoid hamartoma of the maxillary sinus presenting as a periapical radiolucency: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97:607–612. https://doi.org/10.1016/j.tripleo.2003.09.013
Metselaar RM, Stel HV, Van Der Baan S (2005) Respiratory epithelial adenomatoid hamartoma in the nasopharynx. J Laryngol Otol 119:476–478. https://doi.org/10.1258/0022215054273124
Di Carlo R, Rinaldi R, Ottaviano G, Pastore A (2006) Respiratory epithelial adenomatoid hamartoma of the maxillary sinus: case report. Acta Otorhinolaryngol Ital 26:225–227. https://doi.org/10.1258/0022215021910672
Lee JT, Garg R, Brunworth J et al (2013) Sinonasal respiratory epithelial adenomatoid hamartomas: series of 51 cases and literature review. Am J Rhinol Allergy 27:322–328. https://doi.org/10.2500/ajra.2013.27.3905
Nagy AA, Trombitas V, Vlad D, Albu S (2014) Respiratory epithelial adenomatoid hamartoma: a few considerations and a review of literature. Rom J Rhinol 4(15):135–140
Di I, Chirurgia OE, Pastore A (2015) Respiratory epithelial adenomatoid hamartoma of the maxillary sinus: case Respiratory epithelial adenomatoid hamartoma of the maxillary sinus: case report. Int J Case Rep Images 7:346. https://doi.org/10.5348/ijcri-201662-CR-10650
Mulazimoglu S, Islamoglu Y, Beton S et al (2015) Respiratory epithelial adenomatoid hamartoma of the maxillary sinus presenting as an antrochoanal polyp. Egypt J Ear Nose, Throat Allied Sci 16:185–187. https://doi.org/10.1016/j.ejenta.2015.02.006
Shukla A, Kakad T, Shah S, Bhaduri AS (2017) Hamartomatous polyp of the nasopharynx: a rare case report. Indian J Otolaryngol Head Neck Surg. https://doi.org/10.1007/s12070-017-1077-6
Seol JG, Livolsi VA, Malley BWO et al (2010) Respiratory epithelial adnomatoid hamartoma of the bilateral olfactory recesses: a neoplastic mimic? Am J Neuroradiol 31:277–279. https://doi.org/10.3174/ajnr.A1755
Nguyen DT, Nguyen-Thi PL, Gauchotte G et al (2014) Predictors of respiratory epithelial adenomatoid hamartomas of the olfactory clefts in patients with nasal polyposis. Laryngoscope 124:2461–2465. https://doi.org/10.1002/lary.24778
Falco JJ, Peine BS, Clark DW (2017) Bilateral respiratory epithelial adenomatoid hamartomas originating from the anterior olfactory clefts. Baylor Univ Med Cent Proc 30:221–223. https://doi.org/10.1080/08998280.2017.11929594
Delbrouck C, Fernandez Aguilar S, Choufani G, Hassid S (2004) Respiratory epithelial adenomatoid hamartoma associated with nasal polyposis. Am J Otolaryngol Head Neck Med Surg 25:282–284. https://doi.org/10.1016/j.amjoto.2004.02.005
Endo R, Matsuda H, Takahashi M et al (2002) Respiratory epithelial adenomatoid hamartoma in the nasal cavity. Acta Otolaryngol 122:398–400. https://doi.org/10.1080/00016480260000085
Saniasiaya J, Md Shukri N, Ramli RR et al (2017) Sinonasal respiratory epithelial adenomatoid hamartoma: an overlooked entity. Egypt J Ear Nose Throat Allied Sci 18:191–193. https://doi.org/10.1016/J.EJENTA.2016.12.014
Perić A, Bijelić D, Vukomanović-Đurđević B, Perić AV (2016) Huge respiratory epithelial adenomatoid hamartoma originating from the inferior nasal turbinate: a case report. Indian J Otolaryngol Head Neck Surg 68:100–103. https://doi.org/10.1007/s12070-015-0849-0
Cao ZW, Gu ZW, Yang J, Jin MZ (2010) Respiratory epithelial adenomatoid hamartoma of bilateral olfactory clefts associated with nasal polyposis: three cases report and literature review. Auris Nasus Larynx 37:352–356. https://doi.org/10.1016/j.anl.2009.10.003
Lorentz C, Marie B, Vignaud JM, Jankowski R (2012) Respiratory epithelial adenomatoid hamartomas of the olfactory clefts. Eur Arch Oto Rhino Laryngol 269:847–852. https://doi.org/10.1007/s00405-011-1713-9
Hawley KA, Ahmed M, Sindwani R (2013) CT findings of sinonasal respiratory epithelial adenomatoid hamartoma: a closer look at the olfactory clefts. Am J Neuroradiol 34:1086–1090. https://doi.org/10.3174/ajnr.A3345
Vira D, Bhuta S, Wang MB (2011) Respiratory epithelial adenomatoid hamartomas. Laryngoscope 121:2706–2709. https://doi.org/10.1002/lary.22399
Gauchotte G, Marie B, Gallet P et al (2013) Respiratory epithelial adenomatoid hamartoma a poorly recognized entity with mast cell recruitment and frequently associated with nasal polyposis. Am J Surg Pathol 37(11):1678–1685
Prasad ML, Perez-Ordonez B (2009) Nonsquamous lesions of the nasal cavity, paranasal sinuses, and nasopharynx. In: Gnepp DR (ed) Diagnostic surgical pathology of the head and neck. Saunders-Elsevier, Philadelphia, pp 148–154
Mills S (2009) The nose, paranasal sinuses, and nasopharynx. In: Mills S, Carter D, Greenson J et al (eds) Sternberg’s diagnostic surgical pathology, 5th edn. Lippincott Williams & Wilkins, Philadelphia
Acknowledgements
We acknowledge the support of staff and PG students of Department of ENT, Department of Radiology and Department of Pathology, SDM College of Medical Sciences and Hospital, Dharwad.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there are no potential conflicts of interests regarding the publication of this manuscript.
Ethical Approval
All procedures performed in studies involving human participants followed the ethical standards of the institution.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shanbag, R., Patil, P., Rani, S.H. et al. Respiratory Epithelial Adenomatoid Hamartoma (REAH) in the Olfactory Cleft: Often Masked by Bilateral Nasal Polyps. Indian J Otolaryngol Head Neck Surg 71 (Suppl 3), 2121–2126 (2019). https://doi.org/10.1007/s12070-018-1562-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-018-1562-6