Abstract
The objective is to analyze the vestibular system by vestibular evoked myogenic potential (VEMP) in 30 female patients with migraine and balance problem in a controlled study. Thirty female patients with migraine and vestibular problems were enrolled in the study (2009–2012). Fifteen age-matched healthy subjects were selected as the controls. Air conduction cervical VEMP was used. Tone-burst sound stimuli of 95 dB nHL with rarefaction polarity, 5 Hz stimulus repetition rate, 1 ms rise/fall time and 2 ms plateau time were delivered at 500 Hz. 200 sweeps were averaged. Myogenic responses were amplified and band-pass filtered (800–10 Hz). The latency and the amplitude of p1 and n1 waves and interpeak amplitude and latency differences were measured. Results were given as mean and SDs. Interaural p1 and n1 amplitude greater than 30 % asymmetry was accepted as abnormal. VEMP results were compared with controls. The One-way ANOVA test was used. Statistical significance was set at P < 0.05. VEMP responses were elicited in all controls and the patients. Comparative analysis of p1 amplitude between the patients and the controls was statistically significant (P = 0.010). P1n1 interaural amplitude difference was greater than 30 % in 4 patients (13.4 %). No statistically significant difference was found when comparing latency of all wave forms between the patients and healthy controls (P > 0.05). VEMP is an useful tool to test the vestibular system in patients with migraine and balance problem at the very early period. Clinicians should always consider migraine in patients with vertigo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All types of dizziness can be seen in patients with migraine and has been shown to be higher than in the general population [1, 2]. Epidemiological studies suggest that co morbidity of migraine and vertigo is 4.5 % for women and 1.5 % for men [3]. In an analysis of 200 patients from a dizziness clinic, it has been shown that 38 % of patients with dizziness may actually fit into the clinical criteria for migraine [4]. Vestibular migraine is an association between migraine and spontaneous course of vertigo attacks of vestibular origin. Vertigo attacks are often separate from headaches and duration of vertigo often does not fall within the restrictions of aura [5–7]. Idiopathic paroxysmal vertigo of childhood has been defined as a migraine’s equivalent syndrome in which the attacks are brief and have a benign prognosis [8]. Eggers et al. analyzed 228 patients with headache selected from 410 patients with vestibular symptoms and found that those with migraine had distinct vestibular symptoms and presented as recurrent problems [9]. The type of dizziness can be peripheral and central in patients with migraine. Benign paroxysmal positional vertigo (BPPV), Meniere’s disease, motion sickness, cerebellar disorders and anxiety syndromes are more frequent in migraineurs than in controls [3].
Selective testing of the vestibular end-organ was possible after introduction of vestibular evoked myogenic potentials (VEMP) to the clinic by Colebatch and Halmagyi [10, 11]. It is a short latency myogenic wave recorded from the sternocleidomastoid muscle (SCM) after intense acoustic stimulus and is used to assess the integrity and function of the otolithic organ [12]. Abnormal VEMP results have been reported in children with benign paroxysmal vertigo, a migraine variant [13, 14]. Because of highly varied presentation of symptoms, the diagnosis of migraine-related vestibulopathy requires an awareness of presence of vestibular problem due to migraine. The main objective of this study is to review and call attention to impairment of vestibular function in those patients. On the other hand, high prevalence of migraine in women population entails an investigation of a particular relationship between female migraineurs and vertigo. Therefore, we aim to analyze the vestibular system by VEMP in 30 female patients with migraine and balance problem in a controlled study.
Materials and Methods
Thirty female patients with migraine and vestibular problems who had been recruited between 2009 and 2012 were enrolled in the study. They all had a variety of balance problem most recently only (motion sickness, positional unsteadiness, sense of spinning, nausea worsened by head movements or induced by moving objects etc.) and attending to an ENT outpatient clinic for the first time for this problem. A verbal and a signed informed consent was obtained from each patient and 15 control subjects. The procedures were in accordance with the ethical standards of the declaration of Helsinki and of the institutional review board. Those with hearing loss, tinnitus, spontaneous nystagmus, abnormal ear drum, other vestibular or neurologic problems and those who used medication before testing that could affect the vestibular system and/or muscle tone were excluded. All patients had normal otoscopic examination, normal hearing threshold and no problems other than migraine.
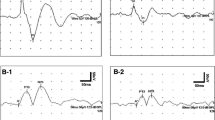
Air conduction cervical VEMP testing was used (Eclipse EP25, Interacoustics, USA) to test the patients. Patients with absence of bilateral VEMPs were excluded. Fifteen healthy subjects with no vestibular problems and intact eardrums were also tested with VEMP as the control group. VEMPs were recorded with disposable, self-adhesive, pre-gelled surface electrodes. Electrode impedances were below 5 kΩ. The active electrode was placed over the middle of the SCM and the reference electrode was placed over the upper sternum. The ground electrode was placed over the forehead. Tone-burst sound stimuli of 95 dB nHL with rarefaction polarity, 5 Hz stimulus repetition rate, 1 ms rise/fall time and 2 ms plateau time were delivered at 500 Hz. 200 sweeps were averaged. Myogenic responses were amplified and band-pass filtered (800–10 Hz). The test was repeated with 85, 75 and 65 dB stimuli in search of the threshold. Stimulation was presented monaurally via an ipsilateral ear phone inserted in foam ear tips (Ear tone-ABR, Interacoustics, USA) while the subjects turned their heads to the counter lateral side in the sitting position and the responses were recorded from the ipsilateral SCM. The test was reviewed twice on each occasion to ensure reproducibility. However, no more than two tests were done and the subjects were allowed to have some rest between trials to prevent fatigue. The procedure was repeated on both sides. Results of VEMP were evaluated by the existence of the first successive p1-n1 biphasic wave. If this was not detected at 95 dB stimulation, the result was accepted as the absence of VEMP. Biphasic VEMP waves seen at 75 or 65 dB stimulation were accepted as VEMP with a lower threshold. Latency and amplitude measurements were made on potentials obtained at 95 dB stimulation.
The latency and the amplitude of p1 and n1 waves and interpeak amplitude and latency differences were measured to determine the magnitude of the whole electrical activity and also to determine whether the myogenic potential is distorted or not. Results were given as mean and SDs. Interaural p1n1 amplitude symmetry was calculated as Lamp (one with larger amplitude)—Samp (one with smaller amplitude)/REamp (Right ear amplitude) + LEamp (Left ear amplitude) × 100. Greater than 30 % asymmetry was accepted as abnormal. VEMP results were compared with controls (Table 1). Patients with no unilateral VEMP response were not included in group analysis. The One-way ANOVA test was used for multiple variance analysis of the groups. Statistical significance was set at P < 0.05.
Results
Age range was between 11 and 51 (33.70 ± 8.87) for the patient’s group and 30–42 (35.25 ± 4.94) for the control group Comparative analysis of average age between the two groups was not statistically significant (P = 0.921). Demographic data of the patients are presented in Table 1. VEMP responses were elicited in all controls and the patients. In the study group, amplitude of VEMP responses was depressed in 4 patients with migraine, that is, the p1n1 interaural amplitude difference was greater than 30 %. Therefore, 4 patients (13.4 %) had a gross VEMP abnormality. Including those patients with VEMP abnormality, comparative analysis of p1 amplitude between the patients and the controls (left ear) was statistically significant (P = 0.010). Comparative analysis of p1n1 amplitude between the patients and the controls (left ear) was not statistically significant, but the difference was striking (P = 0.055). However, no statistically significant difference was found when comparing latency of all wave forms between the patients and healthy controls (P > 0.05). Depression of amplitude was evident in the left ear in all 4 patients. Comparative analysis of p1 amplitude between the right and left ears of patients group was statistically significant due to co-incidence of left ear predominance in all pathologic amplitude values (P = 0.038). Comparative analysis of p1n1 amplitude between the right and left ears of patients group was not statistically significant, but the difference was again striking (P = 0.064). However, no statistically significant difference was found when comparing latency of all wave forms between the right and left ears of patients group (P > 0.05).
Discussion
The underlying pathophysiology of vestibular dysfunction in patients with migraine is obscure although vascular events and ischemic changes appear to be involved. Vasospasm in the posterior circulation has been proposed as a mechanism for migraine-associated damage to the inner ear [15]. Caloric weakness may occur in migraine patients [16, 17]. Von Brevern et al. have reviewed the type of nystagmus during acute attacks of migrainous vertigo. They have reported that 50 % of those had central-vestibular dysfunction, 15 % had peripheral pathology and it was hard to tell the site of involvement in 35 % of them [18]. Liao and Young have found abnormal ENG findings and caloric responses in 30 % of patients and abnormal VEMPs in almost half of the patients with basilar artery migraine [19]. Roceanu et al. have found similar abnormal responses in patients with migraine with or without vertigo [20].
Migraine may affect the vestibular pathway as well as the inner ear. VEMP is a useful tool to test the vestibular end-organ and the sacculocollic reflex pathway which courses through the territory of basilar artery. Baier et al. have reported 68 % reduced VEMP amplitude in 63 patients with vestibular migraine compared to controls. No difference was seen in the latencies and there was no correlation between VEMP amplitudes, tilts of subjective visual vertical (SVV) and caloric testing [21]. Hong et al. have compared VEMP results in 30 patients with migrainous vertigo and 31 healthy volunteers and have found abnormal wave formation in the patient group. However, no difference between groups was observed in latency [22]. Boldingh et al. have analyzed VEMP responses in 37 patients with vestibular migraine and 32 patients with migraine only and they have found that unilateral or bilateral VEMP was absent in 44 % of patients with vestibular migraine and 25 % of migraine [23]. However, contradictory results have also been reported. Kandemir et al. have analyzed 20 patients with migraine without aura, 24 patients with vestibular migraine and 20 patients tension type headache. VEMP latencies and amplitudes were not different from the results of healthy controls although 20.8 % of patients with vestibular migraine had unilateral caloric hypofunction [24].
Our study has shown a statistically significant difference when comparing p1 amplitude between the patients and the healthy control group. The rate of abnormal p1n1 amplitude difference between the ears in the patient group is 13.4 %. This rate of abnormality is somehow lower than those reported in the literature. One possible explanation may be related with the study group. Patients in this study were those who had balance problem for the first time and were those who have been referred to ENT outpatient clinic for initial evaluation. Recurrent or episodic form of their problems have not been documented yet. It is our anticipation that the rate of abnormality will probably increase in those patients during follow-up.
In conclusion, resolution of vestibular symptoms with anti-migraine drugs in patients with migraine-related vertigo as well as a need to initiate preventive therapy provides a challenge for the clinician for the differential diagnosis of this problem from other vestibular pathologies. Occurrence of vertigo with the absence of headache and aura phase of migraine is an important feature that has to be borne in mind especially by ENT clinicians in patients with the first occurrence of unsteadiness. This study shows that patients with migraine associated dizziness may present abnormal results with VEMP. On the other hand, in this study, we aimed a gender specific evaluation due to higher proportion of migraine in women. However, the rate of abnormality was lower. Maybe, it is because their balance problems were new.
References
Cass SP, Furman JM, Ankersteirne K, Balaban C, Yetiser S, Aydogan B (1997) Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 106(3):182–189
Lee H, Sohn SI, Jung DK, Cho YW, Lim JG, Yi SD, Yi HA (2002) Migraine an isolated recurrent vertigo of unknown cause. Neurol Res 24(7):663–665
Lempert T, Neuhauser H (2009) Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 256(3):333–338
Neuhauser H, Leopold M, Von Brevern M, Arnold G, Lempert T (2001) The interrelations of migraine, vertigo, and migraneous vertigo. Neurology 56:436–441
Neuhauser H, Lempert T (1999) Vertigo and dizziness related to migraine: a diagnostic challenge. Cephalalgia 24:83–91
Dietrich M, Brandt T (1999) Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 246:883–892
Brantberg K, Trees N, Baloh RW (2005) Migraine-associated vertigo. Acta Otolaryngol 125:276–279
Marcelli V, Piazza F, Pisani F, Marciano E (2006) Neuro-otological features of benign paroxysmal vertigo and benign paroxysmal positional vertigo in children: a follow-up study. Brain Dev 28:80–84
Eggers SD, Staab JP, Neff BA, Goulson AM, Carlson ML, Shepard NT (2011) Investigation of coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 32(7):1144–1151
Colebatch JG, Halmagyi GM (1992) Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentiation. Neurology 42:1635–1636
Colebatch JG, Halmagyi GM, Skuse NF (1994) Myogenic potentials generated by a click-evoked vestibulocollic response. J Neurol Neurosurg Psychiatr 57:190–197
Basta D, Todt I, Ernst A (2005) Normative data for P1/N1 latencies of vestibular evoked myogenic potentials induced by air- or bone-conducted tone bursts. Clin Neurophysiol 116(9):2216–2219
Zhang D, Fan Z, Han Y, Wang M, Xu L, Luo J, Ai Y, Wang H (2012) Benign paroxysmal vertigo of childhood: diagnostic value of vestibular test and high stimulus rate auditory brainstem response test. Int J Pediatr Otorhinolaryngol 76(1):107–110
Chang CH, Young YH (2007) Caloric and vestibular evoked myogenic potential tests in evaluating children with benign paroxysmal vertigo. Int J Pediatr Otorhinolaryngol 71(3):495–499
Lee H, Whitman GT, Lim JG, Yi SD, Cho YW, Ying S, Baloh RW (2003) Hearing symptoms in migraneous infarction. Arch Neurol 60:113–116
Bir L, Ardic FN, Kara CO, Akalin O, Pinar HS, Celiker A (2003) Migraine patients with or without vertigo: comparison of clinical and electronystagmographic findings. J Otolaryngol 32:234–238
Cutrer FM, Baloh RW (1992) Migraine-associated dizziness. Headache 32:300–304
Von Bravern M, Zeise D, Neuhauser H, Clarke AH, Lempert T (2005) Acute migraneous vertigo: clinical and oculographic findings. Brain 128:365–374
Liao LJ, Young YH (2004) Vestibular evoked myogenic potentials in basilar artery migraine. Laryngoscope 114(7):1305–1309
Roceanu A, Allena M, Da Pasqua V, Bisdorff A, Schoenen J (2008) Abnormalities of the vestíbulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 28:988–990
Baier B, Stieber N, Dieterich M (2009) Vestibular-evoked myogenic potentials in vestibular migraine. J Neurol 256(9):1447–1454
Hong SM, Kim SK, Park CH, Lee JH (2011) Vestibular-evoked myogenic potentials in migraneous vertigo. Otolaryngol Head Neck Surg 144(2):284–287
Boldingh MI, Ljostad U, Mygland A, Monstad P (2011) Vestibular sensitivity in vestibular migraine: VEMPs and motion sickness susceptibility. Cephalalgia 31(11):1209–1211
Kandemir A, Celebisoy N, Kose T (2013) Cervical vestibular evoked myogenic potentials in primary headache disorders. Clin Neurophysiol 124(4):779–784
Acknowledgments
The authors would like to thank to personnel of both clinic for their valuable help.
Conflict of interest
None of the authors have any financial interest for the work or any Grant or financial support provided by companies toward the completion of the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yetiser, S., Gok, M.H., Kutukcu, Y. et al. Vestibular Evoked Myogenic Potentials in a Female Population with Migraine. Indian J Otolaryngol Head Neck Surg 68, 207–210 (2016). https://doi.org/10.1007/s12070-014-0812-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-014-0812-5