Abstract
A visceral artery aneurysm (VAA) is a very rare and lethal vascular anomaly with dramatic consequences. The overall incidence of VAA is 5% of all abdominal artery aneurysms. The involvement of the superior mesenteric artery is even rare (incidence of 3.5–8% of all VAA). The development of superior mesenteric artery pseudoaneurysm following cardiac surgery is scarcely reported in the literature. We report a case of contained rupture of the superior mesenteric artery with no distal flow causing acute mesenteric ischemia (AMI) following double heart valve replacement surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Acute mesenteric ischemia (AMI) is a rare but lethal complication after cardiac surgery. The incidence ranges from 0.4 to 2.9% and is associated with higher mortality (67–100%) [1, 2]. The aetiology of AMI includes superior mesenteric artery embolization, mesenteric venous thrombotic occlusion, and splanchnic vasoconstriction [3]. AMI due to contained rupture of the superior mesenteric artery is very rare following cardiac surgery. We report the case of a patient who developed AMI following double valve replacement due to superior mesenteric artery pseudoaneurysm.
Case report
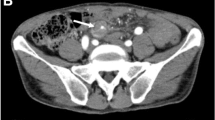
A 25-year-old male underwent double valve replacement surgery at our hospital. Intraoperatively, there was healed vegetation on the aortic leaflet. During surgery, mild hypothermia and blood flow were maintained with a cardiac index of 2.2 L/min. The total bypass time was 110 min, and the surgery was uneventful. Postoperatively, the patient was extubated within 24 h, and he was maintaining good hemodynamics. The postoperative course was uneventful, with no prolonged intensive care unit (ICU) stay and minimal inotropic support. On postoperative day 10, the patient experienced diffuse abdominal pain with abdominal distention and vomiting. Subsequent laboratory findings showed anaemia with the haemoglobin (Hb) of 7 mg/dl, leucocytosis, and raised amylase and lipase levels, suggesting the possibility of acute pancreatitis. To confirm and further delineate the diagnosis, a multidetector computed tomography scan of abdomen was done, which showed a large irregular saccular pseudoaneurysm with a size of 49.5×45.5×59.6 mm after superior mesenteric artery (SMA) origin (Fig. 1); it also showed another 15×13 mm small true aneurysm arising from the jejunal branch of the SMA (Fig. 2). The study revealed fluid-filled dilated few jejunal and ilieal loops and an ascending colon, with subsequent wall enhancement suggesting bowel ischemia and sub-acute intestinal obstruction (Fig. 3). The study also revealed moderate ascites and clots in the pelvis. A gastrosurgical opinion was sought immediately, and the patient was taken for surgical exploration. The patient underwent a successful exploratory laparotomy with resection of the gangrenous small bowel, hemicolectomy, jejulostomy, and ligation of the large pseudoaneurysm of SMA. Histopathologic report showed extensive mucosal necrosis, transmural inflammation, and haemorrhage. The mesenteric blood vessels were congested and thrombosed (Fig. 4) Post exploration, the patient recovered well and maintained good hemodynamics. The patient was put on oral feeds on postoperative day 4, when jejunostomy started functioning. On postoperative day 15, the patient was discharged with overall good hemodynamics and recovery.
Discussion
Visceral artery aneurysm (VAA) is a rare condition, accounting for only 5% of all intraabdominal aneurysms [4]. VAA comprises true aneurysm and pseudo aneurysm, depending on vessel wall involvement. The superior mesenteric artery aneurysm (SMAA) accounts for 3.5–8% of all VAA, whereas the development of pseudoaneurysm is exceedingly uncommon, with an incidence of 0.01–2.6% of all visceral artery pseudoaneurysm (VAPA) [5, 6].
The aetiology of SMA aneurysm and pseudoaneurysm includes various factors, including infection, inflammatory conditions (especially acute pancreatitis), atherosclerosis, collagen vascular disorders, inflammatory disorders, and infrequently, trauma and iatrogenic injury [5]. Based on existing research, it has been observed that a majority of these aneurysms are of mycotic origin, accounting for around 60% of cases, and are commonly associated with sub-acute bacterial endocarditis. Over the years, the development and usage of broad-spectrum antibiotics have led to a reduction in the occurrence of mycotic aneurysms. However, it is pertinent to note that this condition can still develop in patients who are receiving therapy or have already received it [7]. Patients with SMAA may present in various ways from rupture of the artery and causing sudden haemoperitoneum in 30–50% of cases to acute mesenteric ischemia leading to mortality of (67–100%) [8].
Polyarteritis nodosa (PAN) is rare, systemic, necrotizing vasculitis that involves medium-sized arteries. Gastrointestinal complications are prevalent and occur in up to 50% of patients suffering from PAN [9]. The multiple aneurysm formation is most common and involves medium size arteries like a hepatic artery, superior and inferior mesenteric arteries, renal arteries, and gastric artery [10]. Catastrophic bleeding can occur from the rupture of aneurysms, and prompt surgical and catheter-based intervention is required to control the source of bleeding.
Intra-abdominal complications are well mentioned following cardiac surgery. The incidence ranges from 0.5 to 2.6%. The most common being gastrointestinal bleeding followed by bowel ischemia [11]. The development of acute pancreatitis following cardiac surgery can vary depending on diagnostic criteria, and the incidence is estimated between 0.05 and 19% [12]. These complications can be attributed to risk factors like prolonged bypass time, valvular heart surgeries, perioperative hypo-perfusion, low cardiac output state, use of vasopressors, use of an intra-aortic balloon pump, and old age(> 70) [3]. Recognition at the earliest and prompt treatment is the key factor in helping the patients. Diagnosis is best established by computed tomography angiography (CTA).
Various interventions are being used for the treatment of visceral artery aneurysms and pseudoaneurysms, depending on the location of the artery and subsequent organ involvement. Catheter-based techniques are the primary modality of treatment for patients with significant comorbidities and favourable aneurysmal anatomy [13]. Open surgical repair of an artery is a preferred option for ruptured aneurysms with hemoperitoneum and bowel ischemia. The acute mesenteric ischemia due to spontaneous SMA pseudoaneurysm following cardiac surgery was scarcely reported earlier. In our case report, the probable reason is the release of pancreatic autoenzymes in the perivascular space and injury to the arterial wall, resulting in pseudoaneurysm formation [8]. This is a rare case of superior mesenteric artery pseudoaneurysm resulting in acute mesenteric ischemia following cardiac surgery.
Conclusion
Visceral artery aneurysms and pseudoaneurysms are rare complications following cardiac surgery. This case report describes the development of such complication following cardiac surgery.VAPA are more prone to rupture and catastrophic bleeding can occur. In cases where there is suspicion of intestinal ischemia or subacute bowel obstruction, it is imperative to quickly establish a diagnosis and proceed with surgical exploration.
References
Golitaleb M, Golaghaie F, Mousavi MS, Harorani M, Bakhshande Abkenar H, Haghazali M, et al. Gastrointestinal complications after cardiac surgery. Iran Heart J. 2019;20:56–61. https://doi.org/10.1016/s0734-3299(08)70107-2.
Ohri SK, Desai JB, Gaer JA, Roussak JB, Hashemi M, Smith PL, et al. Intraabdominal complications after cardiopulmonary bypass. Ann Thorac Surg. 1991;52:826–31. https://doi.org/10.1016/0003-4975(91)91219-L.
Christenson JT, Schmuziger M, Maurice J, Simonet F, Velebit V. Postoperative visceral hypotension the common cause for gastrointestinal complications after cardiac surgery. Thorac Cardiovasc Surg. 1994;42:152–7. https://doi.org/10.1055/s-2007-1016478.
Chaer RA, Abularrage CJ, Coleman DM, Eslami MH, Kashyap VS, Rockman C, et al. The Society for Vascular Surgery clinical practice guidelines on the management of visceral aneurysms. J Vasc Surg. 2020;72:3S-9S. https://doi.org/10.1016/j.jvs.2020.01.039.
Teixeira PG, Thompson E, Wartman S, Woo K. Infective endocarditis associated superior mesenteric artery pseudoaneurysm. Ann Vasc Surg. 2014;28:1563.e1-1563.e5. https://doi.org/10.1016/j.avsg.2014.03.032.
Lorelli DR, Cambria RA, Seabrook GR, Towne JB. Diagnosis and management of aneurysms involving the superior mesenteric artery and its branches: a report of four cases. Vasc Endovascular Surg. 2003;37:59–66. https://doi.org/10.1177/153857440303700108.
Alvares JF, Parsonnet V, Brief DK. Mycotic aneurysm of the superior mesenteric artery. Am J Surg. 1966;111:237–40. https://doi.org/10.1016/0002-9610(66)90248-0.
Guirgis M, Xu JH, Kaard A, Mwipatayi BP. Spontaneous superior mesenteric artery branch pseudoaneurysm: a rare case report. EJVES Short Rep. 2017;1:1–4. https://doi.org/10.1016/j.ejvssr.2017.09.001.
Tarhan NC, Coskun M, Kayahan EM, Yildirim E, Yucel E. Regression of abdominal visceral aneurysms in polyarteritis nodosa: CT findings. AJR Am J Roentgenol. 2003;180:1617–9.
Lerkvaleekul B, Treepongkaruna S, Ruangwattanapaisarn N, Treesit T, Vilaiyuk S. Recurrent ruptured abdominal aneurysms in polyarteritis nodosa successfully treated with infliximab. Biologics. 2019;13:111–6. https://doi.org/10.2147/BTT.S204726.
Vicari AM, Guisti MC, Luoni R, Shaw PJ, Bates D, CartIidge NEF, et al. Neurologic and nemopsychological morbidity following major surgery. Stroke. 1987;18:892–5.
Chung JW, Ryu SH, Jo JH, Park JY, Lee S, Park SW, et al. Clinical implications and risk factors of acute pancreatitis after cardiac valve surgery. Yonsei Med J. 2013;54:154–9. https://doi.org/10.3349/ymj.2013.54.1.154.
Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G, et al. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45:276–83. https://doi.org/10.1016/j.jvs.2006.10.049.
Funding
U. N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Civil Hospital Campus, Asarwa, Ahmedabad-380016, Gujarat, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nil. The authors declare that they have no conflict of interest.
Informed consent
A written informed consent was obtained from the patient authorising radiological examination, photographic documentation, and surgery. He was also informed that the data concerning the case would be submitted for publication and he consented.
Statement of human and animal rights
There were no infringements of human/animal rights in this investigation.
Ethical committee approval
Since this is a case report, we did not take the ethical clearance as there was no change in the treatment protocol.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, H., Patel, A., Seth, M. et al. A case of contained rupture of the superior mesenteric artery with no distal flow causing mesenteric ischemia following double heart valve replacement surgery. Indian J Thorac Cardiovasc Surg 40, 465–468 (2024). https://doi.org/10.1007/s12055-023-01649-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-023-01649-7