Abstract
Double-outlet left atrium is an extremely rare congenital ventriculo-atrial mal-alignment anomaly, wherein, the left atrium drains into both ventricles, through either a common atrioventricular valve or two separate atrioventricular valves. The only egress from the right atrium is through an inter-atrial communication. We present a 16-month-old male, diagnosed to have double outlet left atrium and describe its surgical management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Double-outlet left atrium (DOLA) is an extremely rare congenital cardiac anomaly, characterized by the left atrium (LA) draining into both the ventricles while the only outlet for the right atrium (RA) is the atrial septal defect (ASD) or a patent foramen ovale (PFO). The morphology of the left atrioventricular valve (AV) can be variable and consensus on an appropriate nomenclature is elusive. Only few such isolated cases and surgical management have been reported in the literature [1,2,3].
Case report
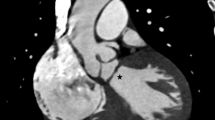
A 16-month-old male child presented with failure to thrive. Hemodynamic parameters were stable with a heart rate of 120 beats/min and respiratory rate of 30/min. He appeared cyanosed with oxygen saturation (SpO2) of 82% on room air. A pan systolic murmur was auscultated at the apex, radiating into the axilla. Electrocardiogram demonstrated left-axis deviation with LA enlargement. Chest radiogram revealed cardiomegaly and pulmonary plethora. Echocardiogram showed RA outlet atresia with PFO serving as the only exit. The LA was draining into both ventricles through a common AV valve. The right component of the AV valve was hypoplastic and the left component had moderate to severe regurgitation, right ventricle (RV) appeared hypoplastic (Fig. 1A). The great arteries were normally related with unobstructed outflow tracts.
The child was considered for the possible complete intracardiac repair and the cavopulmonary connection as an alternative strategy if it fails. A standard cardiopulmonary bypass (CPB) was instituted and the myocardial protection was provided using Del Nido cardioplegia. Upon right atriotomy, no AV valve was seen and the only outlet for systemic venous return was a stretched PFO (Fig. 1B). The RA was severely hypertrophied along with thickened and rightward deviated inter-atrial septum (IAS). The thickened IAS was excised to visualize the common AV valve; the hypoplastic right component opening into RV and a dominant left component opening into the left ventricle (LV). The left AV valve component was severely regurgitant on saline insufflation. Approximation of the left superior and inferior bridging leaflets with interrupted mattress sutures resulted in a competent left component. The ventricular septal defect (VSD) was restrictive and was closed directly with pledgetted sutures. The two components of the common AV valve were separated with autologous pericardium to direct the right and left components into their respective ventricles (Fig. 2A). The pericardial patch at the atrial level was fenestrated in view of hypoplastic right AV valve and RV. Weaning from the CPB was uneventful; however, the central venous pressure (CVP) was 15 mm Hg with SpO2 of 90% on 50% FiO2. Trans-esophageal echocardiogram (TEE) revealed two separate AV valves draining into respective ventricles, right to left shunt across the interatrial patch and gradient (9/6 mm Hg) across the right AV valve (Fig. 2B). In view of these observations, superior cavopulmonary connection was performed. The subsequent saturation was 100% on 50% FIO2 and the gradient across the right AV valve reduced to 6/3 mm Hg.
A Pericardial patch (P) reconstruction of inter-atrial septum with separation of AV valve orifices. L, left AV valve; P, pericardial patch; R, right AV valve; PFO, patent foramen ovale; RA, right atrium; AV, atrio ventricular; B trans-esophageal echocardiogram (TEE) showing reconstructed atrial septum with two separate AV valve opening into their respective ventricles. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; AV, atrio-ventricular
The postoperative course was uneventful. Pre-discharge echocardiogram showed mild flow acceleration across right AV valve component with mild regurgitation, bidirectional shunt across interatrial communication, and trivial left AV valve regurgitation.
Discussion
Embryogenesis of DOLA is highly argued. Van Mierop first described DOLA as a variant of endocardial cushion defect with an assumption that the AV valve represents the common ventriculo-atrial (VA) valve and the abnormality results from the extreme mal-alignment of the atrial septum [4]. However, recent studies of cardiac development suggest that the failure of ingrowth into the developing heart from the dorsal mesenchymal tissues is attributing to the development of this malformation and not the endocardial cushion defect, as thought previously.
Kiraly et al. proposed their hypothesis based on the morphological features of uniatrial biventricular connection. According to them, DOLA like anomaly occurred due to the atresia of the right AV valve and straddling of the left AV valve [5].
As per Van Praagh’s concept, DOLA is considered a ventriculo-atrial malalignment defect resulting from the too far movement of the ventricles and the ventricular septum relative to the atria and the atrial septum during embryogenesis [6].
All patients will have cyanosis due to obligatory mixing of systemic and pulmonary venous returns in the LA. Patients with unrestricted inter-atrial communication will mimic those with isolated atrial septal defects (ASDs), albeit with some cyanosis. It is likely that these patients may miss early diagnosis. However, patients with associated AV valve malformations or inter ventricular communication will present early with features of congestive cardiac failure. Table 1 summarizes the case reports on DOLA.
The goal of surgery is to separate two AV valves by realignment of interatrial septum and correct associated anomalies, if present. If both ventricles are adequate in size, complete excision of deviated IAS and separation of AV valves using a pericardial patch will achieve biventricular repair. If the right AV valve and right ventricle are hypoplastic, as was seen in our case, superior cavopulmonary connection is added.
References
Bhat YA, Pratap H, Dagar KS, Awasthy N. Double-outlet left atrium: successful repair of an extremely rare anomaly. Ann Pediatr Cardiol. 2018;11:204–6.
Shetkar SS, Kothari SS. Double-outlet left atrium: ventriculo-atrial malalignment defect. Ann Pediatr Cardiol. 2013;6:158–61.
Hofbeck M, Wiegand G, Michel J, Schlensak C. Ventriculoatrial malalignment in atrioventricular septal defect resulting in uniatrial biventricular connection: surgical options. J Cardiothorac Surg. 2020;15:59.
Van Mierop LH. Pathology and pathogenesis of endocardial cushion defect: surgical implication. In: Dávila JC, editor. Second Henry Ford Hospital International Symposium on cardiac surgery. New York: Appleton-Century-Crofts; 1977. p. 201–7.
Kiraly L, Hubay M, Cook AC, Ho SY, Anderson RH. Morphologic features of the uniatrial but biventricular atrioventricular connection. J Thorac Cardiovasc Surg. 2007;133:229–34.
Praagh RV. What are double-outlet left atrium and double-outlet right atrium? Ann Pediatr Cardiol. 2013;6:155–7.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Informed written consent was taken from the parents for this publication.
Statement of human and animal rights
Not applicable as no experimental work was carried out.
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, R., Katewa, A., Haranal, M. et al. Double outlet left atrium: successful management of a rare entity. Indian J Thorac Cardiovasc Surg 39, 626–628 (2023). https://doi.org/10.1007/s12055-023-01550-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-023-01550-3