Abstract
Aim
The aim of the study was to assess the role of a temporary carotid shunt in patients undergoing carotid endarterectomy.
Materials and methods
This was a retrospective, multicentric (n = 159) study carried out between January 2005 and October 2020. The study included 3114 patients undergoing carotid endarterectomy who had a reduced retrograde internal carotid artery pressure (<60% of systolic blood pressure). A temporary carotid shunt was used in 1328 patients and 1786 patients underwent carotid endarterectomy without a shunt.
Results
The in-hospital outcomes were comparable in terms of the incidence of deaths, myocardial infarctions, and stroke between the two groups. However, asymptomatic strokes (confirmed on computed tomography) occurred more frequently in the group where the temporary shunt was used (34 (2.5%) vs. 10 (0.55%), p < 0.0001). The composite endpoints of adverse events were also higher in the group where a temporary shunt was used (44 (3.3%) vs. 28 (1.5%), p = 0.002). The risk of symptomatic stroke (both fatal and non-fatal) was higher in the group where a temporary shunt was not used, though this was statistically not significant. Logistic regression analysis identified diabetes mellitus and stenosis (81–90%) of the contralateral internal carotid artery to be important predictors for stroke.
Conclusion
Temporary carotid shunts during carotid endarterectomy were associated with increased rates of asymptomatic stroke. There were no statistically significant differences in the incidence of non-fatal or fatal stroke, myocardial infarction, and mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotid endarterectomy is one of the most commonly performed operations on carotid arteries [1,2,3,4,5]. The safety of carotid endarterectomy depends on reducing the interruption to cerebral blood flow that occurs during clamping of the internal carotid artery (ICA) [6,7,8,9,10]. The assessment of compensatory mechanisms and estimation of the collateral blood flow were carried out previously with the Matas test [11,12,13,14,15]. With technological advancement, the measurement of retrograde pressure in the ICA and cerebral oximetry are now feasible [16,17,18,19,20]. According to current Russian recommendations, use of a temporary shunt during carotid endarterectomy is not mandatory [21,22,23,24,25]. However, there are studies that suggest that when the retrograde pressure in the ICA drops below 60% of the systemic pressure and/or cerebral oximetry parameters fall below 40% of the baseline, a temporary shunt is recommended [26,27,28,29,30]. Temporary shunts are used in a small number of cases of carotid endarterectomy and there is a lack of evidence with respect to their role in reducing postoperative complications [31,32,33,34,35]. The aim of the study was to assess the role of a temporary carotid shunt in patients undergoing carotid endarterectomy with a concomitant decrease in retrograde pressure in the ICA.
Materials and methods
This was a retrospective, multicentric study (n = 159) carried out between January 2005 and October 2020. Retrograde pressure in the ICA was measured in every patient at the time of carotid endarterectomy. Patients who had retrograde pressure in the ICA less than 60% of systolic blood pressure were included in the study.
According to current Russian recommendations, use of temporary carotid shunts, even in the presence of reduced retrograde carotid pressures, is not mandatory. Utilization of a temporary shunt was therefore based on surgeon and institutional preference. Based on the usage of the shunt, two groups were created. Group 1 included patients where a temporary shunt was used, and group 2 where the carotid artery was clamped without using a temporary shunt. The shunt used across all the institutions was the Pruitt-Inahara (LeMaitre) temporary shunt (https://www.lemaitre.com/products/pruitt-f3-carotid-shunts). The choice of the revascularization strategy for the carotid artery was made by a multidisciplinary team that included a cardiovascular surgeon, an endovascular surgeon, a neurosurgeon, a cardiologist, and a neurologist. In the postoperative period, all patients underwent color duplex scanning of the reconstruction area and computed tomography (CT) of the brain.
Outcome of the study
The primary outcome of the study was stroke (fatal, non-fatal, and asymptomatic). Secondary outcomes included myocardial infarction, thrombosis of the ICA, hemodynamically significant restenosis of the ICA, and bleeding — type 3b and higher, according to the Bleeding Academic Research Consortium (BARC) scale. Composite endpoint was defined as sum of both primary and secondarycomplications.
Inclusion criteria
-
1.
All patients eligible for carotid endarterectomy based on current guidelines;
-
2.
Patients with hemodynamically significant stenoses of both the ICAs;
-
3.
Patients where preoperative information was available on the completeness of the circle of Willis based on computed tomography angiography (CTA) of the brain; and
-
4.
Availability of brain CT data in the postoperative period.
Exclusion criteria
-
1.
Contralateral occlusion of the ICA;
-
2.
Contralateral steal syndrome;
-
3.
Open circle of Willis, according to CTA of the brain;
-
4.
Presence of stenosis/occlusion of the vertebral and subclavian arteries;
-
5.
Contraindications to carotid endarterectomy according to current recommendations; and
-
6.
Patients requiring concomitant coronary artery bypass grafting, either as a single or as a staged procedure. (In Russia, the carotidscore.ru calculator is used to select a treatment strategy for such patients).
Carotidscore.ru calculator
Carotidscore.ru is an online risk stratification calculator for postoperative complications of carotid endarterectomy. It was created using a complex mathematical analysis of the results obtained from more than 25 thousand operations carried out in a large Russian multicentric study. Carotidscore.ru helps in identifying patients with a high risk of complications and helps in considering alternative treatment strategies like endovascular intervention or conservative treatment.
Measurement of retrograde pressure in the ICA
When performing carotid endarterectomy in Russia, retrograde pressure in the ICA is measured in all patients. The blood pressure was increased pharmacologically to systolic (180–200 mmHg) and diastolic (90–100 mmHg). Five thousand units of unfractionated heparin was administered intravenously and a direct measurement of retrograde pressure was made in the ICA, distal to the stenosis.
In patients where the retrograde pressure was less than 60% of systemic systolic blood pressure, carotid endarterectomy was performed maintaining a higher blood pressure (systolic between 180 and 200 mmHg and diastolic between 90 and 100 mmHg). If retrograde pressure in the ICA was 60% or more of systemic systolic blood pressure, carotid endarterectomy was performed with blood pressure between 140–179 mmHg systolic and 60–89 mmHg diastolic.
Computed tomography angiography
All patients underwent CTA of intra- and extracranial arteries. The degree of stenosis was determined according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria. Coronary angiography was performed using the Innova 2100 (General Electric, USA) and Innova iQ 3100 (General Electric, USA) units. The severity of coronary lesions was assessed using the SYNTAX Score (www.syntaxscore.com).
Statistical analysis
The type of distribution was determined using the Kolmogorov–Smirnov test. Group comparisons were made using Pearson’s chi-square test with Yates and Mann–Whitney corrections. Differences were assessed as significant at p < 0.05. To identify the predictors of the development of stroke, binary logistic regression was performed with stepwise inclusion and exclusion of predictors (stepwise logistic regression). Statistica for Windows 8.0 was used for data analysis.
Angiography/coronary angiography
The severity of the lesion of the coronary bed corresponded to a mild degree. According to angiography of the brachiocephalic arteries, the groups were comparable in terms of the severity of ipsilateral stenosis of the ICA. At the same time, stenosis of the contralateral ICA was 91–99% statistically more often diagnosed in the group in which a temporary bypass was installed.
Results
Between 2005 and 2020, of all the patients undergoing carotid endarterectomy, 3114 had a retrograde pressure in the ICA less than 60% of the systolic systemic blood pressure and were included in the study. Of these, a temporary shunt was used in 1328 patients (group 1) and 1786 had the procedure without a temporary shunt (group 2).
The groups were well matched in terms of baseline characteristics (Table 1) and had a similar risk profile as confirmed by the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II) (4.9 ± 1.1 vs. 5.0 ± 1.7, p = 0.33). The majority were male and elderly; one in four had a history of myocardial infarction and/or myocardial revascularization. The number of patients presenting with carotid artery stenosis and history of symptomatic stroke was also similar between the groups (Table 1). The incidence of varying degrees of stenosis (91–99%, 81–90%, and 60–80%) in the contralateral ICA was compared between the patients with or without a shunt (Table 2). The incidence of contralateral ICA stenosis 91–99% (near-occlusion) was significantly higher (18.9%) in the group where a shunt was used, compared to the group where a shunt was not used (15.6%), p = 0.01. The duration of clamping of the ICA was similar in both the groups (26.9 ± 3.1 vs. 27.4 ± 3.9, p = 0.36). The average time needed for deployment of the shunt was 46.2 ± 17.6 s.
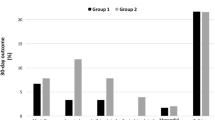
All strokes were ischemic and no hemorrhagic strokes were observed in our study. Overall mortality was 12 (0.3%) and was similar in both groups. Postoperative myocardial infarction (both fatal and non-fatal) and ipsilateral (fatal or non-fatal) stroke and mortality were similar in both the groups (Table 3). The occurrence of asymptomatic ipsilateral stroke was significantly more common in patients where temporary shunt was deployed (p < 0.0001). The composite outcomes, driven primarily by the asymptomatic ipsilateral strokes, were also significantly more common in the group where the shunt was deployed. Diabetes and 81–90% stenosis of the contralateral ICA were independent predictors of all strokes in these patients (Table 4, Fig. 1).
Discussion
There is no consensus on the use of shunts during carotid endarterectomy. On the one hand, indiscriminate use of temporary shunt is associated with the risk of embolization and ischemic stroke, and on the other hand, the failure to use them may lead to cerebral blood flow insufficiency and subsequent adverse events. Proponents of using the shunt have demonstrated both safety and efficacy of the practice [35,36,37,38], while those against the deployment of shunts have reported its futility as well as an increase in incidence of adverse ischemic events [22, 23, 25]. There is evidence that temporary shunt deployment is associated with the development of multiple emboli, often asymptomatic, that result in asymptomatic stroke [26,27,28, 32, 33, 39]. However, currently, there is limited data to either support or discourage the use of shunts during carotid endarterectomy. The choices are guided by surgeon and institutional preferences.
While there is no consensus on use of shunts, there are specific situations where its use is supported by studies. Researchers have used transcranial Doppler to assess the cerebral blood flow and, in the presence of reduced flow, have used temporary shunts with satisfactory outcomes [29]. The protective role of shunts has also been shown in patients presenting with acute watershed stroke and undergoing carotid endarterectomy [30]. Thus, there is some agreement that temporary shunt may be justified in the presence of hemodynamic ipsilateral stroke, as well as in the conditions of unclosed circle of Willis.
It has been argued that routine placement of shunts is inappropriate and may result in complications like dissection, thrombosis, and air embolism. As a result, additional parameters are sought that can guide the usage of shunts. It has therefore been suggested that shunt usage should be guided by cerebral blood flow and should be restricted to patients where there is a potential for compromise. A number of techniques exist to assess cerebral blood flow in this context. However, the more commonly used techniques are near-infrared spectrometry (NIRS) and the stump pressure. NIRS can be used to assess cerebral oxygen levels and if the values after cross-clamp application drops more than 10–20%, a shunt should be deployed. Stump pressure, which reflects the perfusion pressure in the circle of Willis, is an important measure and a value of 30–40 mmHg has been taken as a cut-off. The potential for neurological impairment below this is high. Unfortunately, none of these methods is perfect and some authors have suggested using both the measures to determine placement of a shunt [31].
Our results demonstrated no significant intergroup differences in the development of all symptomatic or clinically apparent neurological adverse events. However, due to routine CT of the brain in the postoperative period, we were able to identify patients with asymptomatic strokes. This was seen mainly in the group where temporary shunt was used. Asymptomatic ischemic strokes were most frequently localized to the frontal lobe and the size was less than 1 cm. The higher composite outcomes, seen in the group where a shunt was used, was driven mainly by the occurrence of asymptomatic strokes. It has been shown that use of temporary shunt has been associated with a large number of microemboli to the brain, which result in these asymptomatic strokes [26,27,28, 40]. The clinical relevance of asymptomatic stroke is however uncertain in the short term, but may have long-term implications.
Another important observation in our work was that the number of clinically apparent or symptomatic strokes were significantly more common in patients where a shunt was not used. This was true for both fatal and non-fatal strokes. However, this was statistically not significant. Our study was large but, because of the event rate (stroke) being low, may not be powered to detect a difference. Linear regression in our study showed that diabetes and stenosis of the contralateral ICA 81–90% were independent risk factors for all strokes. Shunt usage, based on the retrograde measurement of the ICA pressure, was not related to the stroke rate in our study and thus proved to be a low-sensitivity method in predicting hemodynamic cerebral insufficiency, which has also been confirmed by other studies [30, 31].
The independent effect of shunt placement on stroke rates was equivocal in our study and thus the decision to place a temporary shunt should be made based on the experience and preferences of the surgeon, as well as the opinion of a fully informed patient.
Limitations of the study
Retrospective, no randomization.
Conclusion
Placement of a temporary shunt was associated with increased risk of asymptomatic stroke. There was a non-significant increase in symptomatic stroke rates in patients where a shunt was not used, and based on this observation, the authors prefer and recommend the use of a temporary shunt during carotid endarterectomy.
References
Aboyans V, Ricco JB, Bartelink M-LEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. https://doi.org/10.1093/eurheartj/ehx095.
Kazantsev AN, Chernykh KP, Leader RYu, et al. Glomus-sparing carotid endarterectomy according to A.N. Kazantsev. Hospital and mid-term results. Circulatory pathology and cardiac surgery. 2020;24:70–79. https://doi.org/10.21688/1681-3472-2020-3-70-79.
Belov YV, Kazantsev AN, Kravchuk VN, et al. Features of carotid endarterectomy in Russia. How do we Resolution Issues? Curr Probl Cardiol. 2022;47:101272. https://doi.org/10.1016/j.cpcardiol.2022.101272.
Mert B, Mert FTİ, Boyacıoglu K, Sahin I, Ozkaynak B. Carotid artery bypass versus endarterectomy as an alternative treatment of carotid artery stenosis: a propensity score matching analysis. J Stroke Cerebrovasc Dis. 2023;32:106888. https://doi.org/10.1016/j.jstrokecerebrovasdis.2022.106888.
Takahashi T, Uwano I, Akamatsu Y, et al. Prediction of cerebral hyperperfusion following carotid endarterectomy using intravoxel incoherent motion magnetic resonance imaging. J Stroke Cerebrovasc Dis. 2023;32:106909. https://doi.org/10.1016/j.jstrokecerebrovasdis.2022.106909.
Leung YYR, Bera K, Urriza Rodriguez D, et al. Safety of carotid endarterectomy for symptomatic stenosis by age: meta-analysis with individual patient data. Stroke. 2023. https://doi.org/10.1161/STROKEAHA.122.040819.
DeBakey ME. Successful carotid endarterectomy for cerebrovascular insufficiency. Nineteen-year follow-up. JAMA 1975;233:1083-5.
Kazantsev AN, Karkayeva MR, Tritenko AP, et al. Carotid endarterectomy for thrombosis of the internal carotid artery in patients with COVID-19. Curr Probl Cardiol. 2022. https://doi.org/10.1016/j.cpcardiol.2022.101252.
Kazantsev AN, Zharova AS, Sokolova EV, Korotkikh AV. Stenting of the artery of Dr A.N. Kazantsev in the acute period of ischemic stroke. Radiol Case Rep. 2022;17:3699–3708. https://doi.org/10.1016/j.radcr.2022.07.034.
Kazantsev AN, Chernykh KP, Zarkua NE, et al. A new method of glomus-sparing carotid endarterectomy according to A. N. Kazantsev: cutting off the internal carotid artery at the site from the external and common carotid artery. Russ J Cardiol. 2020; 25:10–17. https://doi.org/10.15829/1560-4071-2020-3851.
Jonsson M, Hammar K, Lindberg M, et al. Nationwide outcome analysis of primary carotid endarterectomy in symptomatic patients depending on closure technique and patch type. Eur J Vasc Endovasc Surg. 2023;S1078–5884:00875–9. https://doi.org/10.1016/j.ejvs.2022.12.033.
Yildiz Y, Çiftçi B, Ekinci M, et al. Ultrasound-guided carotid sheath block for carotid endarterectomy surgery in a high-risky patient and literature review. Agri. 2023;35:50–52. English. https://doi.org/10.14744/agri.2021.56987.
Azzam AY, Ghozy S, Elswedy A, et al. Carotid endarterectomy versus carotid stenting for asymptomatic carotid stenosis: evaluating the overlapping meta-analyses of randomized controlled trials. Eur J Radiol Open. 2022;10:100460. https://doi.org/10.1016/j.ejro.2022.100460.
Mohamed A, Shuaib A, Ahmed AZ, Saqqur M, Fatima N. Predictors of 30-day mortality using machine learning approach following carotid endarterectomy. Neurol Sci. 2023;44:253–61. https://doi.org/10.1007/s10072-022-06392-2.
Vuurberg NE, Post ICJH, Keller BPJA, Schaafsma A, Vos CG. A systematic review and meta-analysis on perioperative cerebral and hemodynamic monitoring methods during carotid endarterectomy. Ann Vasc Surg. 2023;88:385–409. https://doi.org/10.1016/j.avsg.2022.08.015.
Gu Y, Zhou Z, Qin Y, et al. Is revascularization of V1 segment of vertebral artery combined with ipsilateral carotid endarterectomy safe? Ann Vasc Surg. 2023;88:218–27. https://doi.org/10.1016/j.avsg.2022.07.020.
Kazantsev AN, Abdullaev IA, Danilchuk LB, et al. CAROTIDSCORE.RU-the first Russian computer program for risk stratification of postoperative complications of carotid endarterectomy. Vascular. 2022:17085381221124709. https://doi.org/10.1177/17085381221124709.
Li B, Eisenberg N, Howe KL, Forbes TL, Roche-Nagle G. The impact of sex on outcomes following carotid endarterectomy. Ann Vasc Surg. 2023;88:210–7. https://doi.org/10.1016/j.avsg.2022.08.003.
Kazantsev AN, Vinogradov RA, Yerofeyev AA, et al. Prolonged atherosclerotic lesion of internal carotid artery: six types of reconstruction. Multiple-center study. Cardiol Cardiovasc Surg. 2021;14:354-369. https://doi.org/10.17116/kardio202114051354.
Noronen K, Söderström M, Kouhia S, Venermo M. Bovine pericardial patch: a good alternative in femoral angioplasty. J Vasc Surg. 2023;77:225–30. https://doi.org/10.1016/j.jvs.2022.08.010.
Kazantsev AN, Chernykh KP, Zarkua NE, et al. Eversion carotid endarterectomy with transposition of the internal carotid artery according to A.N. Kazantsev. Hospital and long-term results. Russian Medical and Biological Bulletin named after Academician I.P. Pavlova. 2021; 29: 73–88. https://doi.org/10.23888/PAVLOVJ202129173-88.
Kazantsev AN, Korotkikh AV, Lider RY, et al. Mathematical model for the choice of tactics of revascularization in case of combined lesions of the carotid and coronary arteries. Curr Probl Cardiol. 2023. https://doi.org/10.1016/j.cpcardiol.2022.101436.
Zaza SI, Bennett KM. The role of patch closure in current-day carotid endarterectomy. J Vasc Surg. 2023;77:170-175.e2. https://doi.org/10.1016/j.jvs.2022.08.003.
De Blasis S, Pulli R, Di Domenico R, et al. Elective or urgent carotid endarterectomy in symptomatic patients: analysis based on the type and timing of neurological symptoms. Ann Vasc Surg. 2022;S0890–5096:00746–4. https://doi.org/10.1016/j.avsg.2022.10.023.
Qumsiyeh Y, Siada S, Yan Y, et al. Carotid endarterectomy is safe for octogenarians. J Vasc Surg. 2023;77:176–81. https://doi.org/10.1016/j.jvs.2022.07.169.
Yei KS, Cui CL, Ramachandran M, Malas MB, Al-Nouri O. Effect of postoperative stroke timing on perioperative mortality after carotid revascularization. Ann Vasc Surg. 2022;S0890–5096:00911–6. https://doi.org/10.1016/j.avsg.2022.12.080.
Rocha-Neves JM, Pereira-Macedo J, Dias-Neto MF, Andrade JP, Mansilha AA. Benefit of selective shunt use during carotid endarterectomy under regional anesthesia. Vascular. 2020;28:505–12. https://doi.org/10.1177/1708538120922098.
Piazza M, Zavatta M, Lamaina M, et al. Early outcomes of routine delayed shunting in carotid endarterectomy for asymptomatic patients. Eur J Vasc Endovasc Surg. 2018;56:334–41. https://doi.org/10.1016/j.ejvs.2018.06.030.
Perini P, Bonifati DM, Tasselli S, Sogaro F. Routine shunting during carotid endarterectomy in patients with acute watershed stroke. Vasc Endovascular Surg. 2017;51:288–94. https://doi.org/10.1177/1538574417708130.
Cho JW, Jeon Y-H, Bae CH. Selective carotid shunting based on intraoperative transcranial Doppler imaging during carotid endarterectomy: a retrospective single-center review. Korean J Thorac Cardiovasc Surg. 2016;49:22–8. https://doi.org/10.5090/kjtcs.2016.49.1.22.
Ceyhan D, Ovali C. The effect of cerebral oximeter use on the shunt placement concerning carotid endarterectomy surgery. Ann Card Anaesth. 2019;22:158–61. https://doi.org/10.4103/aca.ACA_57_18.
Balaji A, Rajagopal N, Yamada Y, Teranishi T, Kawase T, Kato Y. Carotid endarterectomy: the need for in vivo optical spectroscopy in the decision-making on intraoperative shunt usage - a technical note. Asian J Neurosurg. 2019;14:206–10. https://doi.org/10.4103/ajns.AJNS_223_18.
Kazantsev AN, Lider RY, Korotkikh AV, et al. Effects of different types of carotid endarterectomy on the course of resistant arterial hypertension. Vascular. 2022:17085381221140620. https://doi.org/10.1177/17085381221140620.
Kazantsev AN, Korotkikh AV, Lider RY, et al. Computer modeling of carotid endarterectomy with the different shape patches and prediction of the atherosclerotic plaque formation zones. Curr Probl Cardiol. 2023. https://doi.org/10.1016/j.cpcardiol.2022.101505.
Lee J, Lee S, Kim SW, Chang JW. Selective shunting based on dual monitoring with electroencephalography and stump pressure for carotid endarterectomy. Vasc Specialist Int. 2018;34:72–6. https://doi.org/10.5758/vsi.2018.34.3.72.
Chang JW, Kim SW, Lee S, Lee J, Ku MJ. Dual monitoring with stump pressure and electroencephalography during carotid endarterectomy. Korean J Thorac Cardiovasc Surg. 2017;50:94–8. https://doi.org/10.5090/kjtcs.2017.50.2.94.
Makovec M, Kerin K, Skitek M, Jerin A, Klokocovni KT. Association of biomarker S100B and cerebral oximetry with neurological changes during carotid endarterectomy performed in awake patients. Vasa. 2020;49:285–93. https://doi.org/10.1024/0301-1526/a000861.
Coelho A, Peixoto J, Mansilha A, Naylor AR, de Borst GJ. Editor’s choice - Timing of carotid intervention in symptomatic carotid artery stenosis: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2022;63:3–23. https://doi.org/10.1016/j.ejvs.2021.08.021.
Naylor R, Rantner B, Ancetti S, et al. Editor’s choice - European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur J Vasc Endovasc Surg. 2023;65:7–111. https://doi.org/10.1016/j.ejvs.2022.04.011.
Naylor AR. 50 shades of ‘Groundhog Day’. EJVES Vasc Forum. 2022;56:37-39. https://doi.org/10.1016/j.ejvsvf.2022.08.001.
Funding
No funding was received for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical conclusion
The study was performed in compliance with the ethical principles of conducting scientific medical research involving humans and was approved by the decision of the local ethical committee of the Federal State Budgetary Institution of Higher Education “Amur State Medical Academy” of the Ministry of Health of the Russian Federation (protocol no. 3 dated August 25, 2022).
Human and animal rights statement
The study was performed in accordance with the Good Clinical Practice standards and the principles of the Declaration of Helsinki and did not contradict the Federal Law of the Russian Federation of November 21, 2011, No. April 1, 2016, N 200n “On approval of the rules of good clinical practice”. No animals were involved.
Informed consent
Informed consent was obtained from the patients.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kazantsev, A., Korotkikh, A., Lider, R. et al. Results of carotid endarterectomy with the use of temporary shunts with reduced retrograde pressure in the internal carotid artery — analysis of the multicenter Russian register. Indian J Thorac Cardiovasc Surg 39, 244–250 (2023). https://doi.org/10.1007/s12055-023-01487-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-023-01487-7