Abstract
Purpose
Indian patients who undergo surgical revascularization are relatively younger than their Western counterparts and are predominantly revascularized using off-pump coronary artery bypass grafting (OPCAB) technique. They may therefore be at a reduced risk of developing post-operative atrial fibrillation (POAF). The aim of this study was to assess the incidence of POAF, measure its impact on outcomes, and identify the predictors for POAF in the Indian patients undergoing OPCAB. Besides, the ability of European System for Cardiac Operative Risk Evaluation (EuroSCORE) and Society of Thoracic Surgeons (STS) scores in predicting POAF was also assessed.
Methods
In this prospective observational study, all patients undergoing isolated OPCAB in a single institution over a 12-month period were included. Patients undergoing re-operative surgery, emergency procedure, concomitant surgery, or those with history of previously diagnosed or treated atrial fibrillation were excluded. Logistic regression was performed to identify the predictors of POAF. The receiver operating characteristic (ROC) curve was used to determine the ability of EuroSCORE and STS scores to assess risk of developing POAF.
Results
We recruited 1108 patients in the study of which 88 (7.94%) patients developed POAF. Age (OR = 1.082, p < 0.001, 95%CI: 1.050–1.114), unstable angina (OR = 16.32, p = 0.036, 95%CI: 1.2–221.4), presence of diabetes mellitus (OR 1.781, p = 0.025, 95%CI: 1.074–2.955), left atrial size (OR 2.506, p = 0.001, 95%CI: 1.478–4.251), and presence of chronic renal failure (OR 8.7, p = 0.001, 95%CI: 2.4–31.53) were significant predictors of POAF. Both the EuroSCORE (p = 0.035) and the STS score (p = 0.001) were significantly higher in patients developing POAF. The area under the ROC curve for the EuroSCORE II was 0.62 and for the STS score was 0.64 suggesting satisfactory and similar discriminatory power of both the scores to predict POAF in these patients. POAF was associated with significantly increased adverse outcomes like stroke and prolonged hospital stay.
Conclusions
In our study, the incidence of POAF was much lower (7.94%) than that reported previously. POAF significantly increased adverse outcomes and length of hospital stay. Both EuroSCORE II and STS scores had similar discriminating power in predicting POAF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-operative atrial fibrillation (POAF) is seen in nearly one-third of patients undergoing coronary artery bypass grafting (CABG) and has significant impact on patient outcomes like stroke rates, mortality and resource utilization [1, 2]. As a result, several strategies have been attempted to reduce the incidence of POAF after CABG. Pharmacological measures like β-blockers, amiodarone, magnesium, and non-pharmacological measures like atrial pacing and posterior pericardiotomy have all been attempted [3, 4].
Besides avoidance of cardiopulmonary bypass (CPB), the role of off-pump coronary artery bypass grafting (OPCAB) in reducing POAF has also been evaluated by various studies. Even though there are contradictory results in the literature, evidence from randomized comparisons with on-pump CABG and meta-analyses has shown a significant reduction of POAF with the use of OPCAB strategy for surgical coronary revascularization [5, 6].
However, majority of these studies have been carried out in the Western population where the demographics of patients undergoing CABG is considerably different compared to the Indian population. There are a few studies which have evaluated the incidence of POAF in Indian patients [7,8,9,10,11] but often these studies have a small sample size, are retrospective in design, and suffer from significant heterogeneity in terms of operative procedures [8, 10, 11].
Besides evaluating the incidence of POAF in patients undergoing CABG, it is important to identify the predictors of POAF pertaining to the Indian population. The European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the Society of Thoracic Surgeons (STS) score are often used for risk prediction, but their ability to predict the risk of POAF has not been evaluated in the Indian patients [12, 13].
In this prospective study, we sought to examine the incidence of POAF in patients undergoing elective OPCAB. We also attempted to identify the risk factors for POAF as well as evaluated the STS score and EuroSCORE II for a possible association with POAF.
Material and methods
Patients undergoing primary, isolated, elective OPCAB at a single center over a 12-month period (January 2018–December 2018) were enrolled in this prospective observational study. Institutional Ethics Committee approval was obtained (NHRTIICS-EC/AP/2016; 01/08/2016) and informed consent was taken from all study participants. The aim of the study was to determine the incidence of POAF after OPCAB. The other objectives were to identify risk factors for POAF in patients undergoing CABG and the impact it had on post-operative outcomes. Inclusion criteria included all patients undergoing isolated OPCAB during the study period. Exclusion criteria included re-operative surgery, emergency procedure, concomitant surgery, history of previously diagnosed or treated atrial fibrillation (AF), as well as use of CPB, with or without cardioplegic arrest, to perform the CABG.
Definitions
POAF was defined using the STS definition as “AF in the postoperative setting requiring treatment with rate or rhythm control agents, with or without anticoagulation” [14]. Electrocardiographic analysis and continuous monitoring for POAF in patients were done during their stay in the intensive care unit (ICU). Standard 12-lead electrocardiographic recordings were obtained for all patients daily until discharge. Additional electrocardiography recordings are taken if the patient developed new symptoms or signs, such as palpitation, tachycardia, or irregular pulse rhythm. Pre-operative chronic renal failure (CRF) was defined as a glomerular filtration rate < 60 mL/min/1.73 m2. Left ventricular ejection fraction (LVEF) was defined as good (EF ≥ 50%), mild impairment (40–49%), moderate impairment (30–39%), and severe impairment (< 30%). Definitions used in our study with regard to diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), left ventricular (LV) dysfunction, deep sternal wound infection (DSWI), and respiratory and gastro-intestinal (GI) complications were as defined by the STS [15]. Post-operative renal failure was graded according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria, but only stage III (increase of serum creatinine to ≥ 4.0 mg/dL or a 3-fold rise over the most recent pre-operative creatinine level or a new requirement for dialysis) was taken as significant for the purpose of this study. Neurological complications included both cerebro-vascular accident (CVA) and transient ischemic attack (TIA). Respiratory complications included consolidation, pleural effusions, pneumothorax, and prolonged ventilation (> 48 h). Cardiovascular (CVS) complications included high Vasoactive-Inotropic Score (VIS), need for intra-aortic balloon pump, and post-operative myocardial infarction (MI). VIS was defined as high if it was ≥ 10.
Protocol for POAF prevention and management
Beta-blockers were not withdrawn in the pre-operative period and were instituted as soon as possible after the operation. During the ICU stay, 4-hourly potassium measurements were carried out. Magnesium levels were measured if atrial ectopics occurred. Electrical cardioversion was employed where POAF led to significant hemodynamic compromise or if the patient was still ventilated. Drug treatment of POAF was predominantly with amiodarone. In some cases, where the LV function was preserved, sotalol was preferred over amiodarone as a matter of personal preference. Anticoagulation was started if POAF lasted more than 48 h. Only vitamin K antagonists (warfarin or acenocoumarol) were used in the study. The novel oral anticoagulants (NOAC) were not used on any of the patients. We also did not use heparin as a bridging therapy. The target international normalized ratio (INR) was 2.0–3.0 and the duration of therapy was for a minimum of 4 weeks.
Surgical techniques
The strategy of revascularization was OPCAB. Left internal thoracic artery (LITA) was harvested as a pedicle graft in almost all the cases. When bilateral internal thoracic artery (BITA) was used, the right internal thoracic artery (RITA) was harvested as a skeletonized conduit and composite graft was fashioned using the LITA. Radial artery was anastomosed directly to the aorta. There was a general consensus regarding positioning and stabilization techniques during OPCAB, and Octopus (Octopus 3; Medtronic, Minneapolis, MN, USA) and Starfish apical suction devices (Medtronic, Minneapolis, MN, USA) were used for stabilization along with an intra-coronary shunt (ClearView Intracoronary Shunt, Medtronic, Minneapolis, MN, USA).
Statistical analysis
Statistical analysis was performed using SPSS version 20.0. Descriptive statistical analysis was reported using percentage, mean, and standard deviation as appropriate. The chi-square test was used to compare categorical variables between patients who developed POAF and those who did not. Fisher’s exact test was used in case the expected cell frequency was less than 5 in any of the table and chi-square test could not be applied. The Mann-Whitney U test was used to compare ordinal variables between two groups. Spearman’s rank correlation was used to obtain strength of association. For continuous variables, the unpaired t test was used to compare means of continuous variables between patients who developed POAF and those who did not. Receiver operating characteristic (ROC) curve was used to determine ability of EuroSCORE II and STS scores to assess risk of developing POAF. Logistic regression was used to identify predictors of POAF in the study population.
Results
Our study included 1108 patients who underwent elective OPCAB and had a mean age of 59.28 ± 9.11 years. Among the study population, 138 (12.5%) were females, 595 (53.8) were diabetic, and 707 (63.8%) gave history of smoking. A history of previous MI was elicited in 527 (47.6%) patients. The mean EuroSCORE and the STS score in the cohort were 1.89 ± 3.04 and 1.16 ± 1.23, respectively. Of these, 88 (7.94%) cases developed POAF and 1020 remained in sinus rhythm. None of the patients below the age of 40 years had POAF. The rate of POAF in patients between ages of > 40 years but < 55 years was 4% (13 out of 324). This increased to 8.37% (55 out of 657) in patients aged between 55 and 70 years and further increased to 20.6% (20 out of 97 patients) in those aged more than 70 years. There was a weak but positive correlation between age and likelihood of developing POAF (Spearman’s rho = 0.147, p < 0.001). The incidence of POAF in males was higher 82/970(9.23%) compared to females 6/138(4.34%), however, this relationship was statistically non-significant (p = 0.09). Patients with higher New York Heart Association (NYHA) class had significantly higher chance of developing POAF (p < 0.001). Of the 173 patients in class I, only 7 (4%) developed POAF compared to 7.5% (38 out of 501) in class II; 8.9% (37 out of 415) in class III, and 33.3% (6 out of 18) in class IV (Table 1).
With regard to the pre-operative medications, there was no significant difference in the usage of beta-blockers between the 2 groups. The only difference seen was a higher use of pre-operative diuretics in the group that developed POAF (32 (36.4%) vs. 201 (19.7%); p < 0.001) (Table 2). POAF was also significantly more common in patients with CRF (p < 0.001) and those with EF < 30% (p = 0.007) (Table 1). Left atrial (LA) size and left ventricular internal diameter end diastole (LVIDD) were significantly larger in patients who developed POAF. In the group that developed POAF (n = 88), LITA usage was significantly lower (87.5%) compared to those who did not develop POAF (93.8%). The use of radial arteries and usage of RITA were similar in both the groups (Table 3).
POAF was associated with significantly higher adverse outcomes in the post-operative period. The number of patients requiring prolonged ventilation (> 48 hours) was significantly higher in this group along with the post-operative respiratory complications (p < 0.001). Need for re-intubation and re-admission to ICU was also significantly higher (p < 0.001). The CVS complications, as well as stroke rates, were also significantly higher in the group that experienced POAF in the post-operative period (Table 4). There was no difference in 30-day mortality between the groups. At the time of discharge, 22 (25%) patients were on oral amiodarone and 6 (6.8%) were on oral anticoagulation.
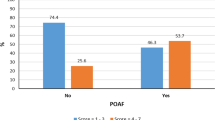
The onset of POAF was within 24 h in 9 (10.2%) cases, which peaked on day 3, when 26 (29.5%) of the patients developed POAF (Fig. 1). Sixteen (18.1%) of the patients developed AF after 1 week in the post-operative period. EuroSCORE II was significantly higher in patients developing POAF (1.88 ± 3.08 vs. 2.55 ± 2.52; p = 0.035). STS score was also significantly higher among patients developing POAF (1.12 ± 1.22 vs.1.59 ± 1.31; p = .001) (Table 5). The ROC curve for the EuroSCORE II showed an area under the curve of 0.62 and for the STS score was 0.64. Thus, there was satisfactory and similar discriminatory ability of both the EuroSCORE II and the STS score to predict post-operative POAF (Fig. 2). A 90% specificity was observed with the STS score of 1.99 and a EuroSCORE II of 2.99.
Age above 60 years (OR 1.082, p < 0.001, CI 1.050–1.114), unstable angina (OR = 16.32, p = 0.036, 95%CI: 1.2–221.4), presence of DM (OR 1.78, p = 0.025, 95%CI: 1.07–2.95), LA size (OR 2.506, p = 0.01, 95%CI 1.47–4.25), and presence of CRF (OR 8.7, p = 0.001, 95%CI: 2.4–31.5) were significant predictors of POAF (Table 6).
Discussion
In our study, pre-operative risk factors like age > 60 years, DM, and LA size were independent risk factors for POAF in patients undergoing OPCAB surgery. POAF did not influence mortality, but significantly increased post-operative adverse events and hospital length of stay. Both STS and EuroSCORE had satisfactory discriminatory power to predict POAF.
The incidence of POAF peaked on the third post-operative day in our study, which is similar to what is reported in the literature [16]. Several factors have been reported to be associated with POAF in patients undergoing cardiac surgery. Pathophysiology of POAF is heterogeneous and can be triggered by inflammation and oxidative stress which lead to increased signal heterogeneity and shortened effective refractory periods [17]. Broadly, cardiac ischemia, electrolyte imbalance, and increased sympathetic activity can all contribute to POAF. Patient-specific factors like age, gender, ethnicity, and body mass index (BMI) are all associated with POAF. Duration of ventilatory support as well as degree of inotropic support can also influence the occurrence of POAF. Abnormalities of LA and LV can also increase the incidence of POAF. Atrial myocardial expression of micro-ribonucleic acid (miRNA) and increased plasma levels of endothelin-1 have been reported to be associated with increased occurrence of POAF [18].
Age is the most consistently reported risk factor for POAF [19,20,21]. It has been shown that there was a progressive increase in the odds of developing POAF with every 10-year increase in age [22]. In our study, the risk of POAF was 21% in patients older than 70 years of age. Several pathophysiological mechanisms have been proposed for age-related increase in POAF. There is an increase in comorbidities with age, which interact in a synergistic fashion. There is decreased compliance of both the left ventricle and left atrium with age which results in higher filling pressure. These higher filling pressures contribute to atrial stretch, which in turn increases the propensity to develop AF [23]. Further evidence of age being the single most independent risk factor for POAF is provided by the fact that it is included in all risk prediction scores that have attempted to predict the risk of POAF. The CHA2DS2-VASc score starts the scoring at a cut-off age of 65 years, the POAF score uses a cut-off of 60 years, and the HATCH score (hypertension < 1 point >, age > 75 years < 1 point >, transient ischemic attack [TIA] or stroke < 2 points>, chronic obstructive pulmonary disease [COPD] < 1 point >, and heart failure < 2 points >) and the combined AF (COM-AF) score both use 75 years as the cut-off [12, 24,25,26].
DM emerged as an independent risk factor for developing POAF in our study. There is ample evidence in the literature confirming this association. It has been shown that patients with DM have a 40% higher risk of POAF compared to non-diabetic patients and the risk of developing POAF increases by 3% every year in diabetic patients [27]. While studies have reported DM to be an important risk factor for POAF in patients undergoing CABG, there is scarcity of data in the specific cohort of patients undergoing OPCAB surgery [28]. A number of physiological mechanisms have been proposed for the association between diabetes and AF. Patients with DM have been reported to have a higher level of C-reactive protein suggesting a pro-inflammatory state. This inflammatory milieu leads to myocardial fibrosis and diastolic dysfunction. DM is also known to influence the autonomic system, as well as causes structural remodelling, thus influencing the occurrence of POAF [20]. In the presence of coronary artery disease, coronary microvascular dysfunction has been suggested as another potential mechanism [29]. The importance of DM in the pathophysiology of AF has led to its inclusion in the CHADS2 and the CHA2DS2-VASc scoring systems [25].
The effect of LA size has been unreported in most studies. However, studies which examined the influence of LA size on rates of POAF have reported increased LA size to be a risk factor for POAF [19, 30,31,32,33]. LA size serves as an indirect marker for the severity and chronicity of LV diastolic dysfunction and may explain the increased incidence of atrial arrhythmias [34]. The remodelling and resultant dilation of the atria predispose to re-entrant electrical circuits and POAF. Unstable angina and CRF were also found to be independent risk factors for developing POAF. However, a wide confidence interval was observed with both these variables. This could have resulted due to only 255 (23%) of patients presenting with unstable angina and a mere 13 (1.1%) patients with CRF. Evidence exists about a bi-directional relationship between chronic kidney disease and AF [35]. In the context of cardiac surgery, renal dysfunction has been shown to be an independent risk factor for POAF [36]. The mechanisms by which renal dysfunction leads to POAF are not completely elucidated. Some of the proposed mechanisms include pathological activation of the renin-angiotensin-aldosterone system that results in myocardial fibrosis, LV diastolic dysfunction, and increase in LA enlargement and stretch. This leads to electrical as well as structural remodelling of the left atrium, which increases the risk of POAF [36]. In some cases, where the left anterior descending (LAD) artery was perceived to be a very poor target, LITA was preferentially grafted to the diagonal or even the circumflex territory. In other cases, intra-operative hemodynamic concerns may have led to saphenous vein graft (SVG) being used for the LAD anastomosis. Thus, the SVG-LAD anastomosis could be seen as a surrogate marker for a higher risk profile and perhaps was a confounder. This was confirmed on our analysis, where this association was seen only on univariate analysis. After adjusting for the risk scores and other variables, the SVG-LAD was no longer associated with increased incidence of POAF.
The incidence of POAF was lower in our series (7.9%) compared to contemporary reported studies. There are several important reasons for this finding. First and foremost, our study population comprised younger patients with one-third of the patients below the age of 40 years. None of the patients below 40 years developed POAF. Moreover, we followed a very strict protocol, where beta-blockers were continued until CABG and were initiated as soon as possible in the post-operative period. While beta-blockers provide considerable protection against POAF, they have been shown to be more effective in younger patients, which was the case in our study [37]. All our patients were operated using the OPCAB technique, which is the strategy of myocardial revascularization in > 50% of patients in India [38]. There is some controversy whether OPCAB is truly protective against POAF compared to on-pump CABG. A study carried out in an Indian population showed a non-significant increase in the rate of POAF in patients undergoing CABG using CPB (17.2%) versus OPCAB (12.9%) [39]. A meta-analysis examining this question concluded that OPCAB is protective in younger patients, but not in the elderly [5]. Thus, a combination of diligent use of beta-blockers and utilization of OPCAB technique of myocardial revascularization in a young cohort of patients was probably responsible for the low POAF rates seen in our study.
POAF results in a significant prolongation of length of stay in the hospital and leads to increased utilization of resources [40]. There has been evidence of POAF being associated with increased long-term mortality. However, POAF has not been shown to increase in-hospital mortality and stroke rates and is in keeping with the findings of our study [32]. POAF was associated with higher EuroSCORE II and STS scores. There have been some studies which have examined the association between EuroSCORE II and POAF [20, 39]. Both EuroSCORE and STS scores had similar correlation with POAF as seen by largely similar area under the curve (AUC) of the ROC curves and future risk prediction models could consider including these scores.
Limitations
Our study population was limited to OPCAB surgery, and the risk factors identified may not be generalizable to on-pump CABG or extrapolated for other cardiac surgical procedures. The patient population in our study was younger than most reported series and are unlikely to be replicated in the elderly population. Our definition of POAF included only those AF which needed treatment and longer-term outcomes were not assessed. Post-operative respiratory, renal, and cardiac complications can all increase the occurrence of POAF. They can also be triggered by these events. As a result, a definite cause and effect relationship is difficult to establish in these cases. Our study did not include a control arm where CPB was used. As a result, the findings of our study cannot be used as evidence of OPCAB being protective against POAF. Moreover, it was a single-center study, and a multi-center study design would be required to address regional variations.
Conclusions
In our study on Indian patients, the incidence of POAF (7.94%) was much lower than that reported previously. None of the patients below the age of 40 years experienced POAF. Occurrence of POAF significantly increased adverse outcomes and length of stay in hospital. Both EuroSCORE II and STS scores had satisfactory and similar discriminatory power for POAF.
References
Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. https://doi.org/10.1093/eurheartj/ehy394.
Kosuma P, Wachirasrisirikul S, Jedsadayanmata A. Attributable costs of postoperative atrial fibrillation among patients undergoing cardiac surgery. Cardiol Res Pract. 2018;2018:3759238. https://doi.org/10.1155/2018/3759238.
Arsenault KA, Yusuf AM, Crystal E, et al. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;2013:CD003611. Published 2013 Jan 31. https://doi.org/10.1002/14651858.CD003611.pub3.
Gaudino M, Sanna T, Ballman KV, et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single-centre, single-blind, randomised, controlled trial. Lancet. 2021;398:2075–83. https://doi.org/10.1016/S0140-6736(21)02490-9.
Athanasiou T, Aziz O, Mangoush O, et al. Does off-pump coronary artery bypass reduce the incidence of post-operative atrial fibrillation? A question revisited. Eur J Cardiothorac Surg. 2004;26:701–10. https://doi.org/10.1016/j.ejcts.2004.05.053.
Wu C-Y, Wang S-H, Shang Y-Q, Xia J-H. Incidence of atrial fibrillation after off-pump versus on-pump coronary artery bypass grafting: a meta-analysis of randomized clinical trials and propensity score matching trials. J Huazhong Univ Sci Technolog Med Sci. 2017;37:956–64. https://doi.org/10.1007/s11596-017-1834-5.
Agarwal GR, Krishna N, Raveendran G, et al. Early outcomes in patients undergoing off-pump coronary artery bypass grafting. Indian J Thorac Cardiovasc Surg. 2019;35:168–74. https://doi.org/10.1007/s12055-018-0730-3.
Dave S, Nirgude A, Gujjar P, Sharma R. Incidence and risk factors for development of atrial fibrillation after cardiac surgery under cardiopulmonary bypass. Indian J Anaesth. 2018;62:887–91. https://doi.org/10.4103/ija.IJA_6_18.
Ghurram A, Krishna N, Bhaskaran R, Kumaraswamy N, Jayant A, Varma PK. Patients who develop post-operative atrial fibrillation have reduced survival after off-pump coronary artery bypass grafting. Indian J Thorac Cardiovasc Surg. 2020;36:6–13. https://doi.org/10.1007/s12055-019-00844-9.
Krishna VR, Patil N, Nileshwar A. Prospective evaluation of the utility of CHA2DS2-VASc score in the prediction of postoperative atrial fibrillation after off-pump coronary artery bypass surgery - an observational study. Ann Card Anaesth. 2020;23:122–6. https://doi.org/10.4103/aca.ACA_161_18.
Sarin K, Chauhan S, Bisoi AK, Kapoor PM, Gharde P, Choudhury A. Relationship between perioperative left atrial appendage Doppler velocity estimates and new-onset atrial fibrillation in patients undergoing coronary artery bypass graft surgery with cardiopulmonary bypass. Ann Card Anaesth. 2017;20:403–7. https://doi.org/10.4103/aca.ACA_73_17.
Mariscalco G, Biancari F, Zanobini M, et al. Bedside tool for predicting the risk of postoperative atrial fibrillation after cardiac surgery: the POAF Score. J Am Heart Assoc. 2014;3:e000752. https://doi.org/10.1161/JAHA.113.000752.
Pollock BD, Filardo G, da Graca B, et al. Predicting new-onset post-coronary artery bypass graft atrial fibrillation with existing risk scores. Ann Thorac Surg. 2018;105:115–21. https://doi.org/10.1016/j.athoracsur.2017.06.075.
Frendl G, Sodickson AC, Chung MK, et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg. 2014;148:e153–93. https://doi.org/10.1016/j.jtcvs.2014.06.036.
Adult Cardiac Surgery Database Data Collection | STS. Accessed February 3, 2022. https://www.sts.org/registries-research-center/sts-national-database/adult-cardiac-surgery-database/data-collection
Frost L, Mølgaard H, Christiansen EH, Hjortholm K, Paulsen PK, Thomsen PE. Atrial fibrillation and flutter after coronary artery bypass surgery: epidemiology, risk factors and preventive trials. Int J Cardiol. 1992;36:253–61. https://doi.org/10.1016/0167-5273(92)90293-c.
Matos JD, Sellke FW, Zimetbaum P. Post-cardiac surgery atrial fibrillation: risks, mechanisms, prevention, and management. Card Electrophysiol Clin. 2021;13:133–40. https://doi.org/10.1016/j.ccep.2020.11.011.
Qureshi M, Ahmed A, Massie V, Marshall E, Harky A. Determinants of atrial fibrillation after cardiac surgery. Rev Cardiovasc Med. 2021;22:329–41. https://doi.org/10.31083/j.rcm2202040.
Ferreira AF, Saraiva FA, Moreira R, et al. Postoperative atrial fibrillation after coronary artery bypass grafting surgery. Rev Port Cir Cardiotorac Vasc. 2017;24:129.
Narayan P, Mandal CK, Das R, et al. Atrial fibrillation - can HbA1c levels really predict the risk? Asian Cardiovasc Thorac Ann. 2022;30:141–146.
Omar A, Elshihy EM, Singer M, Zarif D, Dawoud O. Perioperative risk factors predisposing to atrial fibrillation after CABG surgery. Heart Surg Forum. 2021;24:E402–6. https://doi.org/10.1532/hsf.3759.
Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–9. https://doi.org/10.1001/jama.291.14.1720.
Bhave P, Passman R. Age as a risk factor for atrial fibrillation and flutter after coronary artery bypass grafting. J Atr Fibrillation. 2012;4:482. https://doi.org/10.4022/jafib.482.
Burgos LM, Ramírez AG, Seoane L, et al. New combined risk score to predict atrial fibrillation after cardiac surgery: COM-AF. Ann Card Anaesth. 2021;24:458–63. https://doi.org/10.4103/aca.ACA_34_20.
Chua S-K, Shyu K-G, Lu M-J, et al. Clinical utility of CHADS2 and CHA2DS2-VASc scoring systems for predicting postoperative atrial fibrillation after cardiac surgery. J Thorac Cardiovasc Surg. 2013;146:919–926.e1. https://doi.org/10.1016/j.jtcvs.2013.03.040.
de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725–31. https://doi.org/10.1016/j.jacc.2009.11.040.
Dublin S, Glazer NL, Smith NL, et al. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med. 2010;25:853–8. https://doi.org/10.1007/s11606-010-1340-y.
Tadic M, Ivanovic B, Zivkovic N. Predictors of atrial fibrillation following coronary artery bypass surgery. Med Sci Monit. 2011;17:CR48–55. https://doi.org/10.12659/msm.881329.
Erdogan D, Akcay S, Yucel H, et al. The effects of good glycaemic control on left ventricular and coronary endothelial functions in patients with poorly controlled type 2 diabetes mellitus. Clin Endocrinol (Oxf). 2015;82:388–396. https://doi.org/10.1111/cen.12520.
Gode S, Aksu T, Demirel A, et al. Effect of vitamin D deficiency on the development of postoperative atrial fibrillation in coronary artery bypass patients. J Cardiovasc Thorac Res. 2016;8:140–146. https://doi.org/10.15171/jcvtr.2016.29.
Ismail MF, El-Mahrouk AF, Hamouda TH, Radwan H, Haneef A, Jamjoom AA. Factors influencing postoperative atrial fibrillation in patients undergoing on-pump coronary artery bypass grafting, single center experience. J Cardiothorac Surg. 2017;12:40. https://doi.org/10.1186/s13019-017-0609-1.
Konstantino Y, Zelnik Yovel D, Friger MD, Sahar G, Knyazer B, Amit G. Postoperative atrial fibrillation following coronary artery bypass graft surgery predicts long-term atrial fibrillation and stroke. Isr Med Assoc J. 2016;18:744–8.
Luo W, Huaibin W, Wenjun Z, et al. Predictors of postoperative atrial fibrillation after isolated on-pump coronary artery bypass grafting in patients ≥60 years old. Heart Surg Forum. 2017;20:E038–42. https://doi.org/10.1532/hsf.1583.
Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1961–77. https://doi.org/10.1016/j.jacc.2019.01.059.
Ding WY, Potpara TS, Blomström-Lundqvist C, et al. Impact of renal impairment on atrial fibrillation: ESC-EHRA EORP-AF Long-Term General Registry. Eur J Clin Invest. 2022:e13745.
Chua S-K, Shyu K-G, Lu M-J, et al. Renal dysfunction and the risk of postoperative atrial fibrillation after cardiac surgery: role beyond the CHA2DS2-VASc score. Europace. 2015;17:1363–70.
Fuller JA, Adams GG, Buxton B. Atrial fibrillation after coronary artery bypass grafting. Is it a disorder of the elderly? J Thorac Cardiovasc Surg. 1989;97:821–5.
Sajja LR. The journey of surgery for coronary artery disease in India: adoption, customization and innovation. Indian J Thorac Cardiovasc Surg. 2014;30:116–28. https://doi.org/10.1007/s12055-014-0282-0.
Sajja LR, Singh S, Mannam G, Guttikonda J, Pusapati VRR, Saikiran KVSS. Impact of occult renal disease on the outcomes of off-pump and on-pump coronary artery bypass grafting. Indian J Thorac Cardiovasc Surg. 2019;35:150–7. https://doi.org/10.1007/s12055-018-0767-3.
Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–7. https://doi.org/10.1161/01.cir.94.3.390.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights statement
It is confirmed that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Ethics committee approval
EC approval was obtained [NHRTIICS-EC/AP/2016; 01/08/2016].
Informed consent
Informed consent was obtained from all patients included in the study.
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Potdar, S.P., Shales, S., Baviskar, M. et al. Incidence, predictors, and outcome for post-operative atrial fibrillation in Indian patients undergoing off-pump coronary artery bypass grafting—a prospective observational study. Indian J Thorac Cardiovasc Surg 38, 366–374 (2022). https://doi.org/10.1007/s12055-022-01358-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-022-01358-7