Abstract
Purpose
Hybrid aortic arch replacement (HAAR) is emerging as a safe treatment alternative for aortic arch pathologies. HAAR is divided into three groups. We have assessed our outcome for all three types of HAAR.
Method
From January 2007 to December 2016, we have performed 119 endovascular aortic repair (EVAR) of the aorta of which 56 were hybrid aortic arch repair. The hybrid repair entailed aortic arch vessel debranching and concomitant/delayed antegrade ± retrograde EVAR stent grafting of the arch. For group I and II hybrid patients, we debranch the supra-aortic arch vessels without the aid of circulatory arrest. EVAR was performed on the following day. In group III, hybrid antegrade EVAR of the thoracic aorta and arch reconstruction was performed in single stage.
Results
Of the 56 patients, 16 were in group I, 32 in group II, and 8 in group III. Mean age was 59.9 ± 9.4 years with 78.57% (n = 44) being males. Aortic dissection was the primary pathology in 31 (55.36%) patients followed by aneurysm in 24 (42.86%) patients. Marfans syndrome was present in 28.57% (n = 16) patients. Redosternotomy was performed in 10.71% patients (n = 6). Incidence of stroke was 5.38% (n = 3) and there was no patients with renal dysfunction requiring hemodialysis. There were two retrograde aortic dissections and two endoleaks, both in group I patients. Thirty days in-hospital mortality was 5.38% (2 in group I and 1 in group II).
Conclusion
Hybrid aortic arch replacement can be performed with good postoperative outcome. Type II hybrid is better than type I hybrid in our experience. As experience increases, the outcome continues to improve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of aortic arch pathologies remains a challenge even in the present era. When diseases of the aorta such as the aneurysms, aortic dissection, or pseudo aneurysm involve the aortic arch, open aortic arch replacements are done, which will require a deep hypothermic circulatory arrest. Neurological and cardiovascular complications still remain a significant cause of morbidity and mortality in open surgical repair (OSR), even with the improved techniques over the last two decades [1, 2]. This is even more evident in high-risk candidates—such as old age and patients with high comorbidity index. The influx of stents grafts for thoracic endovascular aortic repair (TEVAR) has provided an alternate to complex aortic arch pathologies, especially in high-risk cohorts. “Hybrid” aortic arch repair (HAAR)—an amalgamation of conventional surgical techniques and endovascular technology—has minimised the risk of operation. It seeks to limit operative, bypass, and circulatory arrest time by simplifying and shortening the arch repair procedure. Hybrid arch procedures necessitate two main doctrines: (i) efficient debranching of the supra-aortic arch branches—innominate artery, left common carotid artery (LCCA), and left subclavian artery (LSA) and (ii) creation of adequate proximal and distal landing zone (LZ) for the TEVAR. We are presenting our experience with HAAR, with early and midterm follow-up.
Methods
From January 2007 to December 2016, 119 patients underwent endovascular aortic repair (EVAR). Of these, 56 underwent “Hybrid” aortic arch repair. They fall into three groups—groups I, II, and III.
Group I
These are group of patients (16 of the 56) who had isolated aortic arch pathology. The stent graft is landed proximally on native aorta either in zone 0 or zone 1 (Fig. 1).
Group I cohort. a Isolated aneurysm of the distal aortic arch. b A carotid–carotid bypass was performed and the endograft was landed in zone 1. c Type B aortic dissection with an entry point in the distal aortic arch. d Debranching of the innominate and left common carotid arteries were done from the native ascending aorta. Endograft was landed in zone 0
Zone 0 – Native aorta: Debranching of the supra-aortic arch branches were achieved by median sternotomy. The stent graft was deployed covering these branches, with proximal landing on zone 0. Debranching is usually done with an inverted Y graft or straight grafts without the aid of cardiopulmonary bypass (CPB). As these are inherently weakened aorta, it is advisable to apply the side-biting clamp on the ascending aorta at a blood pressure less than 100 mmHg. We momentarily clamp the inferior vena cava to temporarily reduce the blood pressure.
Zone 1 – Proximal Aortic arch: In these patients, the pathology was confined to the distal aortic arch, but with 2 cm or more of proximal landing zone distal to innominate artery. An extra-anatomical carotid–carotid and left carotid–left subclavian artery bypass was performed in the neck, following which a stent graft was deployed covering the LCCA and beyond. The LCCA is ligated in the neck proximal to the anastomosis creating a functional end-to-end anastomosis.
Group II
Thirty-two patients received this mode of treatment (Fig. 2). These patients had a native ascending aorta which was unsuitable for landing the stent graft. Ascending aorta was replaced with a Dacron graft and debranching of the supra-aortic arch branches was performed—thus creating a “Dacron zone 0” for landing the graft. Based on the aortic root anatomy, a root replacement - aortic valve replacement or repair were performed. At our centre, we replace the ascending aorta when the size is more than 4 cm. This is because we noted these patients have an inherited weakened aorta, thus increasing the chance of retrograde aortic dissection or presenting with ascending aortic aneurysm during follow-up.
Group II cohort. a Aortic arch aneurysm involving the mid and distal aortic arch. b Ascending aorta was replaced and a Y-graft used to debranch the innominate and the left common carotid artery. Endograft was landed in Dacron zone 0. c Type B aortic dissection in a young male. d Debranching of the innominate and the left common carotid artery was done with two separate vascular grafts after replacing the ascending aorta. Endograft was landed in Dacron zone 0
Group III
Of the 56 patients, 8 received this type of repair. In these patients, under fluoroscopic guidance, a stiff guide wire was advanced from the groin and stationed in the ascending aorta. Care was taken that the wire was in the true lumen in its entire course. Cardiopulmonary bypass was established through the axillary or innominate artery. Cardiac arrest was obtained with cardioplegia. Under moderate hypothermia (24 °C), aortic arch was opened and bi-carotid selective antegrade cerebral perfusion was initiated. The distal aorta was prepared, approximating the true and false lumen. Antegrade TEVAR was performed to the proximal descending thoracic aorta over the guide wire. The stent graft was then sutured onto the proximal descending thoracic aorta. A four branch aortic arch graft was then sutured to the stent graft. Through the side arm of the arch graft, lower body perfusion was initiated. Supra-aortic arch branches were then anastomosed back to the arch graft (Fig. 3).
Group III cohort. a Patient underwent Bentall’s procedure for type A aortic dissection elsewhere, presented to our hospital with residual type B aortic dissection. b Aortic arch replacement with antegrade endovascular stenting to the descending thoracic aorta was performed. c In a 45-year Marfan’s patients, Bentall procedure using cabrol technique to LMCA was performed by us. Ten years late, he presented to us with type B aortic dissection. d Aortic arch replacement with antegrade endovascular stenting to the descending thoracic aorta was performed
Indication for left subclavian artery preservation
The indications to preserve the LSA include dominant left vertebral artery, occluded or absent right vertebral artery, patent left internal mammary artery, anatomical anomaly of left vertebral artery arising directly from the aortic arch, patients on hemodialysis who require access to fistula or functioning arterio-venous fistula, patent left axillo-femoral bypass graft, and /or evidence of left arm ischemia after stent graft coverage [3, 4]. The LSA is persevered in group I patients by an extra-anatomical LCCA to LSA bypass and by direct reimplantation in group II and group III patients.
Statistical analysis and follow-up methods
All statistical comparisons were done using SPSS 19 (SPSS Inc., Chicago, II). Cumulative survival was calculated using Kaplan–Meier analysis. All patients were followed postoperatively, with regular contrast CT Aortogram imaging (discharge, 6 months, and yearly). Follow-up was 100% in all groups. Institutional review board approval was obtained. Log rank test was used to compare the survival curves and a p value of < 0.05 was considered to be statistically significant. For survival analysis, the ‘survival’ package of R version 3.4.0 was used [5].
Results
From January 2007 to December 2016, 56 patients have received HAAR at our centre. The demographic pattern is shown in Table 1. Mean age was 59.9 ± 9.4 years and 78.57% (n = 44) being males. Aortic dissection (acute and chronic) was the presenting complaint in 55.36% (n = 31), aneurysmal disease of the aorta was present in 42.86% (n = 24) of patients, and 1 patient underwent treatment for pseudo aneurysm following trauma. 3.57% (n = 2) had previous history of stroke, 8.93% (n = 5) had coronary artery disease, and 8.93% (n = 5) had renal dysfunction. More than one fourth of the patients (28.57%, n = 16) were diagnosed to have Marfan’s syndrome. One tenth of the patients (n = 6) who had previous cardiac procedures required a re-sternotomy – 2 aortic valve replacement, 1 – ascending aorta replacement, 2- Coronary artery bypass grafting, 1 – atrial septal defect closure.
All 16 patients in group I were done without the aid of cardiopulmonary bypass, using a side-biting clamp on the ascending aorta for supra-aortic arch vessel debranching. The mean timing during cardiopulmonary bypass, cross clamp, and antegrade perfusion for each group is shown in Table 2. Retrograde TEVAR was performed in all group I patients (femoral approach) and antegrade deployment of TEVAR was done in all group III patients. In group II patients, antegrade approach was used in patients who had Bentall’s procedure (5 patients); in others, the endograft was deployed via the femoral approach (Fig. 2).
The 30-day/in-hospital mortality was 3/56 (5.25%) for the entire cohort (Table 3). Mortality was 12.5% (2/16) for the group I, and 3.12% (1/32) for the group II and zero for group III patients. Causes of both deaths in group I was due to retrograde aortic dissection. Late type II endoleaks occurred in two patients, both in group I. Both these patients required reintervention with endovascular procedure to treat the same. There were no endoleaks in group II and group III patients. Stroke was presented in three patients—two in group I and one in group II. The patients in group II had previous history of cerebrovascular accident. There was no stroke, spinal cord ischemia, renal failure, endoleak, or 30-day mortality in group III patients.
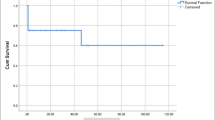
The event-free survival for group I patients at 100, 200, 300, 400, and 500 weeks was 71, 64, 57, 57, and 57% respectively. Hence, at 300 weeks after the procedure, 43% of the patients in group I had some event including the death. On the contrary, the event-free survival for group II patient at 100, 200, 300, 400, and 500 weeks was 93, 93, 93, 93, and 93% respectively. Hence, in these patients if the initial procedure goes well, they have an event-free survival for 500 weeks (Fig. 4a).
a The event-free survival for group I patients at 100, 200, 300, 400, and 500 weeks was 71, 64, 57, 57, and 57% respectively. The event-free survival for group II patient at 100, 200, 300, 400, and 500 weeks was 93, 93, 93, 93, and 93% respectively. The Kaplan–Meier curve shows a greater event-free survival in group II and is statistically significant. b The overall survival (taking death alone as a variable) for group I patients at 100, 200, 300, 400, and 500 weeks was 87, 87, 76, 76, and 76% respectively. The overall survival for group II patient at 100, 200, 300, 400, and 500 weeks are 97, 97, 97, 97, and 97% respectively
The overall survival (taking death alone as a variable) for group I patients at 100, 200, 300, 400, and 500 weeks was 87, 87, 76, 76, and 76% respectively. Hence, at 300 weeks after the procedure, one quarter of the patient in the cohort was dead. The overall survival for group II patient at 100, 200, 300, 400, and 500 weeks are 97, 97, 97, 97, and 97% respectively (Fig. 4b).
Discussion
In 1990s after the introduction of antegrade cerebral perfusion in arch procedure, there has been a significant improvement in the outcome with conventional operative management. In the present day, HAAR is being performed with increasing frequency for the treatment of aortic arch pathology. The Achilles heel of all the aortic arch interventions remains neurologic complications. HAAR has offered a less-invasive approach and an early favourable result [6,7,8,9]. The complications associated with HAAR have not been fully understood, but there is a continuous evolvement in the technology of endografts and the technique in deployment.
Several groups have shown that HAAR can be performed with acceptable morbidity and mortality. In a systemic review of 1886 patients by Cao et al. [10], the pooled mortality was 10.8% and stroke risk was 7%. The perioperative mortality was higher in disease extending to the ascending aorta (15.1%), whereas there was no significant difference in the neurological outcome. The pooled mortality in frozen elephant trunk ranged from 9.8 to 13.2%. The author has also shown that there is an outcome difference between the procedure performed before and after 2007, favouring the latter. The neurological outcomes were positively affected by the high volume index of the centre and the experience of the operator.
No randomised controlled studies do exist to compare HAAR and OSR. But the potential advantages of a hybrid repair technique are as follows: (1) absence of deep hypothermia, circulatory arrest (group I/II), and hence avoiding associated coagulopathy/haemorrhage /cerebral ischemia; (2) arch reconstruction is made into an easier—a more “proximal”—operation; (3) in case of aortic dissection complete exclusion of primary tear with improved recognition and treatment of acute malperfusion; (4) elimination of residual tears in the arch and proximal DTA which are a risk factor for aortic complications in the future; (5) provides a suitable proximal LZ, if future distal endovascular treatment is required; and (6) can be performed in elderly high-risk patients in whom major aortic procedure can prove deleterious [11].
The Ishimaru’s classification scheme defines five TEVAR landing zones. Each zone is bound by an imaginary line aligned with distal line of each of the supra-aortic arch vessel [12].
-
Zone 0: Involves the origin of the innominate artery
-
Zone 1: Involves the origin of the LCCA
-
Zone 2: Involves the origin of the LSA
-
Zone 3: The proximal descending thoracic aorta down to the T4 vertebral body
-
Zone 4: The reminder of the thoracic aorta
Frozen elephant trunk grafts (Jotec E-Vita, Vascutek Thoraflex, and certain custom made grafts) are not available in southern Asia. Hybrid operating theatre is still not a verisimilitude in a country like India, probably due to cost of establishing the same. In such situations, a mobile fluoroscopy unit, thoracic endograft, and four branch vascular aortic arch grafts can be used as a proxy to perform the same surgery with comparable results. Such surrogates make these surgeries feasible in the developing countries in addition to having a cost benefit.
However, as is the case with of every new technique, problems do arise. Two pathologies which arose especially following EVAR are retrograde type A aortic dissection (rAAD) and endoleaks. The incidence of rAAD is 1.33%, but bears a high mortality rate of 42% [13]. The probable mechanism can be as a result of the compliance mismatch between the highly elastic ascending aorta and the still rigid stent graft, altered hemodynamic and tangential clamp injury on an inherently weakened aorta [13]. This disaster can be avoided by creating a “Dacron zone 0” for landing the graft and a prophylactic replacement of the ascending aorta when it is 40 mm or more in diameter. All endeavours should be aimed to minimise manipulation of guide wire and stent graft systems. Eggebrecht et al. has also shown that rAAD can also be diagnosed soon after the procedure and even up to 1050 days after the procedure and more than one fourth of these patients are asymptomatic [13]. Hence, this calls to attention the life-long surveillance of these HAAR patients.
Endoleaks are characterised by the persistent blood flow within the aneurysmal sac following the endograft. Depending up on the cause they have been classified into five types.
-
Type I: Leak at the graft attachment site (proximal or distal)
-
Type II: Aneurysmal sac filling via a branch vessel
-
Type III: Leak through defect in the graft
-
Type IV: Leak through graft fabrics as a result of graft porosity
-
Type V: Endotension
Literature report on incidence of endoleaks in HAAR ranges from 0 to 15% in various studies. Similar to TEVAR, even in HAAR, type I and III endoleaks are associated with greater morbidity than type II endoleaks. Incidence of endoleak is higher in patients who had the endograft landed in zone 1 compared to zone 0; also, the reintervention rates are higher in patients who had the stent stationed in zone 1 [14]. Recently Cao et al. in meta-analysis have pointed out those two elements which determine the results for HAAR: the level of emergency surgery and the landing zone for the prosthesis. It is also shown that there is a twofold increased mortality for implantation in zone 0 versus zone 1 (15.1 vs. 7.6%) [10].
In our study, the immediate postoperative outcomes showed statistical significance between group I and group II patients, favouring group II. Hence, we prefer to replace the ascending aorta and create a “Dacron zone 0” for the stent graft. This possibly prevents the chances of rAAD. There is a shorter landing zone when the graft is stationed in zone 1. Further, this shorter LZ along with steeper angulation of the aortic arch may lead to poorer anchoring of the stent graft in zone 1. This may be further exacerbated during the positive remodelling of the aorta. These can explain the higher rates of endoleak in group I patients. Based on our experience, we now preform more of type II repair than type 1 repair (Fig. 5).
Our experience corroborates to the learning curve involved with the HAAR, and with experience, even the more complex arch hybrid procedures can be performed with low mortality and morbidity, and good midterm outcomes. Meticulous preoperative planning and coordination with team work is the sum and substance of a successful hybrid aortic arch repair.
References
Kim T, Martin TD, Lee WA, et al. Evolution in the management of the total thoracic aorta. J Thorac Cardiovasc Surg. 2009;137:627–34.
Milewski RK, Szeto WY, Pochettino A, Moser GW, Moeller P, Bavaria JE. Have hybrid procedures replaced open aortic arch reconstruction inhigh-risk patients? A comparative study of elective open arch debranching with endovascular stent graft placement and conventional elective open total and distal aortic arch reconstruction. J Thorac Cardiovasc Surg. 2010;140:590–7.
Hughes GC, Daneshmand MA, Swaminathan M, et al. "Real world" thoracic endografting: results with the Gore TAG device 2 years after U.S. FDA approval. Ann Thorac Surg.2008;86: 1530–1538.
Clough RE, Modarai B, Topple JA, et al. Predictors of stroke and paraplegia in thoracic aortic endovascular intervention. Eur J Vasc Endovasc Surg. 2011;41:303–10.
Therneau T (2015). A Package for Survival Analysis in S.r package version 2.38, <https://CRAN.Rproject.org/package=survival>).
Matalanis G, Durairaj M, Brooks M. A hybrid technique of aortic arch branch transposition and antegrade stent graft deployment for complete arch repair without cardiopulmonary bypass. Eur J Cardiothorac Surg. 2006;29: 611–2.
Kpodonu J, Diethrich EB. Hybrid interventions for the treatment of the complex aortic arch. Perspect Vasc Surg EndovascTher. 2007;19:174–84.
Shin JH, Yoon HK, Chung CH, et al. Hybrid procedure with antegrade stentgraft placement for aortic arch aneurysms: preliminary experience in eight patients. J Vasc Interv Radiol. 2011;22: 148–54.
Lee CW, Beaver TM, Klodell CT Jr, et al. Arch debranching versus elephant trunk procedures for hybrid repair of thoracic aortic pathologies. Ann Thorac Surg. 2011;91:465–71.
Cao P, De Rango P, Czerny M, et al. Systematic review of clinical outcomes in hybrid procedures for aortic archdissections and other arch diseases. J Thorac Cardiovasc Surg. 2012;144:1286–300.
Appoo JJ, Pozeg Z. Strategies in the surgical treatment of type A aortic arch dissectionAnn Cardiothorac Surg.2013;2:205–211.
Kotelis D, Geisbüsch P, Attigah N, Hinz U, Hyhlik-Dürr A, Böckler D. Total vs hemiaortic arch transposition for hybrid aortic arch repair. J Vasc Surg. 2011;54:1182–6.
Eggebrecht H, Thompson M, Rousseau H, et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation.2009;120:S276–81.
Lin J, Marrocco C, Galovich J, et al. Experience with early TEVAR treatment of uncomplicated type B aortic dissection. J Cardiovasc Surg. 2013; 54 :161–72.
Acknowledgements
We acknowledge Dr. Suresh V, Faculty, Department of community medicine, SRMC&RI, Chennai, for helping us with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This is a retrospective study involving human and has been cleared by our Institutional Ethical Committee.
Conflict of interest
The author declares that they have no conflict of interest.
Informed consent
NA
Rights and permissions
About this article
Cite this article
Idhrees, M., Krishnaswami, M., Jacob, A. et al. Hybrid aortic arch repair: 10-year experience from India. Indian J Thorac Cardiovasc Surg 35 (Suppl 2), 156–163 (2019). https://doi.org/10.1007/s12055-018-0689-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-018-0689-0