Abstract
Considerable progress has been made in the field of lung cancer surgery since the first sleeve lobectomy was performed 70 years ago. Technology has advanced significantly and a multitude of lung cancer treatments and diagnostic tests is now available. These advancements have led to improved surgical treatments and understanding of this disease. Historically, pneumonectomy was considered the procedure of choice for central or locally advanced non-small cell lung cancer (NSCLC). Sleeve lobectomy was conceived only as an alternative for patients with limited cardiopulmonary reserve. However, the advantages of bronchoplastic resections for NSCLC have long been established (Thomas et al. J R Coll Surg Edinb. 1956;1:169–186; Allison et al. Ann R Coll Surg. 1954; 25:20–22; Thomas et al. Thorax. 1960;15:9–11; Weisel et al. J Thorac Cardiovasc Surg. 1979;78(6):839–849; Jensik et al. J Thorac Cardiovasc Surg. 1972;64(3):400–412. Many reliable studies have published convincing results over the years demonstrating superior outcomes over pneumonectomy when sleeve lobectomy is extended to patients with normal cardiopulmonary reserve (Gaissert et al. Thorac Cardiovasc Surg. 111(5):948–953, 1996). Not only does sleeve lobectomy surgery preserve lung function, but literature over the years has clearly shown this procedure to be associated with comparable morbidity, decreased mortality, and favorable long-term survival and enhanced quality of life (Weisel et al. J Thorac Cardiovasc Surg. 1979;78:839–849; Jensik et al. J Thorac Cardiovasc Surg. 1972;64:400–412, Ferguson et al. Ann Thorac Surg. 2003;76:1782–1788). In this review, we highlight patient selection factors and current indications for considering sleeve lobectomy. In addition, we describe our sleeve lobectomy surgical techniques and review post-operative management strategies and potential associated complications. We also discuss the long-term outcomes, recurrence, and survival rates associated with sleeve lobectomy in more recently available published series.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the paucity of patients undergoing sleeve lobectomy compared to pneumonectomy, sleeve lobectomy surgery for non-small cell lung cancer (NSCLC) originating in the lobar bronchus is well established and is now the preferred and curative method to managing central airway tumors in surgical candidates [1, 2]. This conclusion has evolved over time and sleeve lobectomy was initially thought of as an alternative to pneumonectomy for managing central endobronchial tumors, in patients with impaired cardiopulmonary reserve. Sleeve lobectomy can now be safely performed from both open and minimally invasive approaches, and even following induction chemotherapy [3,4,5,6,7]. A very recent published series demonstrated the safety of robotic sleeve lobectomy for lung cancer in 21 patients with a mortality rate very similar to that reported of open series [8]. We continue to recognize, based on much experience, that sleeve lobectomy should be considered a primary intervention in lieu of pneumonectomy.
The first notable sleeve lobectomy was performed in 1947 by Price-Thomas, and several years later, Allison reported the first use of sleeve lobectomy in carcinoma treatment [9,10,11]. In 2008, Boffa et al. reviewed the Society of Thoracic Surgeons general thoracic surgery database and the surgical management of primary lung tumors. They reported that between 1999 and 2006, out of the 9033 pulmonary resections analyzed, there were 591 pneumonectomies and only 85 sleeve lobectomies completed for primary lung cancer (6.5 vs 0.9%). The reported operative mortality in the pneumonectomy group was 6.2% and the 30-day mortality 5.3%, compared to lobectomy and sleeve group with a reported 2 and 1.8%, respectively [1]. The increased operative risk observed in pneumonectomy compared with that of sleeve lobectomy and reported 11 years ago still stands today [1].
The benefits of sleeve lobectomy have been well established [12, 13]. In addition to being more cost effective and preserving pulmonary function, sleeve lobectomy when compared with pneumonectomy can be completed with a much lower risk of operative and overall mortality. It is also associated with improved local control and long-term survival and enhanced quality of life. Today, we expect the operative mortality for sleeve lobectomy to be less than 5%. [14]. The most recent large series by Pages et al. examining overall and disease-free survival rates associated with sleeve lobectomy in 942 patients showed a 71.9% overall 3-year survival after sleeve lobectomy compared to 60.8% after pneumonectomy. In addition, 3-year disease-free survival in the sleeve group was 46.4 and 31.6% in the pneumonectomy group. The pneumonectomy group also showed an increased risk of disease recurrence in comparison to the sleeve group [15]. The superior outcomes noted in this study of sleeve lobectomy patients with NSCLC should strongly encourage surgeons to embrace this surgical approach in similar patient populations.
In this paper, we review the patient selection factors and current indications for considering sleeve lobectomy. In addition, we describe our sleeve lobectomy surgical technique and review post-operative management strategies and potential associated complications. We also discuss the outcomes and survival rates associated with sleeve lobectomy in more recently available published series.
Considerations for patient selection
According to the Surveillance, Epidemiology, and End Results (SEER) database, the estimated number of new lung cancer cases in the USA is 222,500, with the disease most frequently being diagnosed in people aged 65–74 years old. Many of these patients will have tumors with involvement of the central airways at the time of diagnosis, in the form of endobronchial tumor extension, bulky disease, or compressive peribronchial lymphadenopathy. Often, partial or complete airway obstruction will limit functional status in these patients and cause dyspnea, hemoptysis, or cough. Many patients will go undiagnosed for some time and may only have vague symptoms on presentation. Tumor occurring in the lobar orifice or invading the main bronchus precludes standard surgical lobectomy, and in these cases, whenever R0 resection is feasible, sleeve lobectomy should be considered as the procedure of choice and considered equivalent to a pneumonectomy. Even in patients with N1 disease, sleeve lobectomy has been shown to have superior outcomes when compared to pneumonectomy [5, 6, 16, 17]. For every patient presenting with complex lung cancer, an assessment of operability should be expeditious and thorough, as surgical resection can often be curative in patients with central airway disease. In brief, the indications for sleeve lobectomy include the following: patients diagnosed with central lung cancer; a lobar resection margin positive for malignancy on frozen section; avoidance of pneumonectomy in patients where preservation of lung parenchyma is key, and those with compromised cardiopulmonary function.
Operability of patients with central airway tumors should be assessed by an interdisciplinary expert team involving thoracic surgeons, pulmonologists, cardiologists, radiologists, pathologists, oncologists, and radiation oncologists. This multidisciplinary team approach to lung cancer patients in general is also imperative to long-term follow-up and life-long surveillance. Detailed imaging and cardiopulmonary function testing is essential for determining candidacy for sleeve lobectomy or pneumonectomy and planning the operative strategy. It is reasonable to obtain an echocardiogram and cardiac stress test, in addition to pulmonary function testing on all patients being considered for this surgery. Patient selection for sleeve lobectomy thereafter will ultimately depend on tumor location, tumor type, and accurate preoperative nodal staging. The central airways should be assessed initially with non-invasive computed tomography (CT) imaging to evaluate for airway obstruction, narrowing, potential surgical margins, pulmonary artery involvement, associated nodal enlargement, and relationship of adjacent parenchymal lung masses. A CT angiogram can assist with planning for possible concomitant pulmonary artery resection if involvement is suspected. Three-dimensional reconstruction of images can help visualize disease with greater certainty and identify planes of potential dissection. Fiberoptic bronchoscopy is recommended to evaluate endobronchial extent of disease and can often be utilized for diagnostic purposes also. Tissue sampling for diagnosis is almost always possible with bronchoscopy for proximal endobronchial tumors and can be complimented with the use of navigational bronchoscopy and radial endobronchial ultrasound (EBUS) when needed. CT-guided lung biopsies of the primary lesion can be reliably used when diagnosis is called into question with these approaches. Once a diagnosis is firmly established, a positron emission tomography (PET)-CT scan should be obtained for complete staging purposes and to ensure no evidence of disease which would preclude resection. This can also be useful in determining the need of EBUS-transbronchial needle aspiration (TBNA) or mediastinoscopy for nodal sampling prior to lobectomy. Induction therapy when indicated is recommended in an effort to downstage locally advanced central tumors and encourage later resection. Either chemotherapy alone or concurrent chemoradiation, using a platinum-based combination therapy, is typically chosen. Although preoperative chemoradiotherapy can contribute to the complexity of the surgery, studies have shown improved long-term survival rates observed in patients who underwent sleeve lobectomy even after induction therapy when compared with pneumonectomy [18]. In a multicenter analysis by Cusumano et al. in 2014, the 5-year survival rates following sleeve lobectomy for NSCLC in 51 patients who received induction therapy was 53.8% [5]. Maurizi et al. reported on 82 patients in 2013 who had undergone sleeve lobectomy after induction therapy. There were no post-operative mortalities and no significant differences noted in post-operative complications when compared to those in the sleeve lobectomy patients without induction therapy [19]. Analysis of long-term survival rates according to stage and nodal status has shown that sleeve lobectomy typically results in higher survival rates for stages I and II [18]. There is no known survival benefit of performing a pneumonectomy in patients with N2 disease over a sleeve lobectomy [2].
Operative approach
Although sleeve lobectomy surgery is performed predominantly for right upper lobe lesions, the surgical principles can be applied for any lobar resection. Once it is decided by preoperative cardiopulmonary testing, clinical staging, and preoperative planning that a patient is a reasonable candidate for a sleeve lobectomy, the operative strategy should be carefully thought out. We will typically perform a thorough bronchoscopic evaluation of the airways, followed by an endobronchial ultrasound EBUS-TBNA or mediastinoscopy for N2 nodal assessment, within the same setting as the sleeve lobectomy procedure. Ipsilateral N2 disease may result in the patient being referred for neoadjuvant therapy with reassessment of operability after treatment. N2 disease does not absolutely contraindicate resection. We typically clinically restage after therapy with PET-CT. If there is no evidence of distant disease on subsequent PET-CT, we proceed with lung resection without restaging the mediastinum surgically. If multistation N2 disease is found, patients are given definitive chemoradiation and surgery is not offered. Since our sleeve resection rate in positive N2 disease is quite low, we have not compared outcomes of neoadjuvant vs adjuvant therapy in our institution. However, in 2013, Gonzalez et al. reported no significant differences noted in 90-day mortality and morbidity after sleeve resection for patients treated with and without induction therapy, including patients with N2 disease [20].
Once operability has been confirmed, it is typical to proceed with side-specific double-lumen endotracheal tube intubation for single lung ventilation. We recommend starting each case with a surveillance video-assisted thoracoscopic surgery (VATS) to ensure resectability and no evidence of metastatic disease. A single 5-mm incision made at the seventh or eighth posterior intercostal space facilitates placement of a single port and a 5-mm thoracoscopic camera to carry out an initial examination of the pleural space.
If a three-port VATS approach to sleeve lobectomy is planned thereafter, then the previous incision is extended to accomodate our 10-mm flexible-tip thoracoscope with high-definition picture quality. A second incision is made at the sixth intercostal space as anteriorly as possible, in line with the major fissure, in order to facilitate stapler placement during hilar structure division. Articulating and curved tip staplers can decrease vascular injury in our experience [21]. A 3–4-cm access incision at the midaxillary line is made at the fourth interspace for upper lobectomies and at the fifth interspace for lower lobectomies. A self-retaining wound-protector is typically placed to improve exposure and to decrease trauma to the adjacent tissues during thoracoscopic instrument placement. Mahtabifard et al. discussed the first series of VATS sleeve lobectomy procedures in 2008 in 13 patients. They showed that this procedure, though technically challenging, could be performed through a minimally invasive approach with results corresponding closely to open surgery. Since then, several authors have discussed outcomes of minimally invasive VATS sleeve lobectomy and current surgical approaches have now extended to include robotic sleeve lobectomies [3, 4, 7].
For open-sleeve lobectomies, a standard posterolateral serratus-sparing, at the fourth or fifth intercostal incision is made. The intercostal muscle is harvested as a pedicle and divided anteriorly and set aside for use in bronchial stump coverage later. The use of a vessel-sealing energy device or electrocautery is used to divide the inferior pulmonary ligament, anterior, and posterior mediastinal pleura. Once tumor resectability is confirmed, the hilum is exposed. We recommend careful dissection and control of the main pulmonary artery early on in preparation for pulmonary artery reconstruction as needed. Opening the pericardium can facilitate this dissection when a tumor is obstructing access. The pulmonary artery is encircled with a long vessel loop and an appropriately sized vascular clamp is set aside in anticipation of the need to obtain vascular control during dissection.
After sleeve lobectomy feasibility is determined, the venous and arterial branches are dissected and divided in a routine sequential manner. Frozen section bronchial margin analysis is paramount to sleeve lobectomy operation. In the event of a positive resection margin, additional tissue should be resected until a disease-free bronchial margin is assured. It is important to note that all bronchi should be divided perpendicular to the long axis of the airway. We use a red-rubber catheter to encircle the airways to allow easier passage of the stapling device. Devascularization of the bronchus is avoided in an attempt to minimize post-operative bronchial complications.
If pulmonary artery reconstruction is anticipated, we typically give 5000 U of intravenous heparin prior to clamping the main pulmonary artery. We will also place a vessel loop around the inferior pulmonary vein to minimize backflow during reconstruction. The bronchial anastomosis is completed first to avoid tension on the artery. Primary end-to-end arterial anastomosis can be completed when possible using a 5-0 prolene running suture or subsequent use of a bovine pericardial reconstructive patch. Side-biting vascular clamps can facilitate reconstruction nicely. We do not reverse the heparin and will continue patients on anticoagulation for 30 days if a pulmonary artery reconstruction is performed. As mentioned previously, although right upper lobe sleeve lobectomies are the most commonly performed, sleeve lobectomy with or without pulmonary artery involvement can be performed safely in all the lobes.
Important technical considerations include avoiding tension on the anastomosis and kinking of the pulmonary artery and vein. Lymph node stations should be cleared after completing the lobectomy in an effort to accurately stage the patient. A bronchoscopy is performed prior to chest closure to ensure adequacy of the anastomosis and patency of the reconstructed airway. Below, in (Tables 1, 2, 3, 4, and 5), we review the main operative steps taken for each sleeve lobectomy. With main stem tumors, a complete lung-sparing sleeve resection can be performed in select instances. The appended movie (video 1) demonstrates one such case.
(M4V 86048 kb)
Post-operative care
An intra-operative multilevel intercostal nerve block is performed for every thoracic surgery at our institution using liposomal bupivacaine. This has significantly decreased the amount of post-operative pain patients experience and the use of intravenous narcotics has been minimized. Epidural use is infrequent since the implementation of liposomal-based bupivacaine injection. Judicious use of intravenous fluids and diuretics are used in an effort to avoid post-operative pulmonary edema. Routine beta blockers are given in an attempt to minimize arrhythmias. A single chest tube is left in place and typically placed on water seal on the first post-operative day, with removal once any air leak has resolved. Patients are extubated immediately following the surgical procedure and pulmonary hygiene is emphasized. Inhaled mucolytic agents and nebulizers are prescribed routinely along with aggressive incentive spirometry and chest physiotherapy so as to mobilize secretions early in the post-operative course. Bronchoscopy is utilized on an as-needed basis for poor mucocilliary clearance and atelectasis. Patients are ambulatory immediately after surgery and daily physical therapy is standard. Length of stay has been evaluated in several studies. The average length of stay in our institution for an uncomplicated sleeve lobectomy patient is estimated at 5 days.
Potential complications
Potential complications reported after sleeve lobectomy in patients have been reported in many series with morbidities comparable to those noted after pneumonectomy. The most commonly reported complications are listed in Table 6 and include prolonged air leak, arrhythmias, pneumonia, and atelectasis. Sleeve lobectomy and pneumonectomy for patients with N2 and stage III disease was reported by Deslauriers et al. in 2004, indicating that pneumonectomy does not improve survival in patients with a more advanced disease [22]. Induction therapy can be considered in this group. As demonstrated in studies by Maurizi, Bagan, and Milman listed in Table 7, induction therapy has been shown to have no significant impact on procedure-related morbidity or mortality [19, 23, 24].
Recurrence and survival
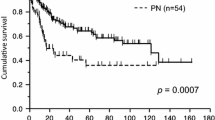
An important consideration for selecting sleeve resection over pneumonectomy is its safe completion with a potential of achieving at least equivalent oncological results. The studies comparing the locoregional recurrence and 5-year survival of sleeve lobectomy (SL) and pneumonectomy (PN) are shown in Table 8. With the exception of Maurizi et al., all other studies listed include patients with or without neoadjuvant therapy; the former including only the patients who had received neoadjuvant chemotherapy. Merritt et al., in a non-comparative study, reported a 44% overall 5-year survival rate in their NSCLC sleeve lobectomy patient population of 196 patients, and 39.3% in the patients with N1 disease [16]. With only few institutions performing VATS sleeve resections (with or without robot assistance), most of the literature available on this approach is relatively recent with reference to only short-term outcomes.
Discussion
Despite continued encouraging results, sleeve lobectomy remains significantly underutilized worldwide. It is not always understood as the standard of care for anatomically suitable central airway cancers and pneumonectomy procedures continue to outnumber sleeve lobectomies. The studies comparing sleeve resections and pneumonectomies have shown an improved overall 5-year survival for the sleeves, comparable locoregional recurrence rates, and no increase in morbidity and mortality after induction therapy (Tables 7 and 8). This supports the oncologic validity of sleeve resections. The noted improved overall survival may be due to better cardiopulmonary status post-operatively in the sleeve groups. Owing to thoracic remodeling after pneumonectomy, we also expect lesser chronic pain issues after sleeve resection. With VATS pneumonectomy (with and without robot assistance), we have previously shown significantly more pain-free patients at 1 year compared to open approach [33]. As more literature for the VATS approach becomes available, it would be an interesting comparison of chronic pain between the VATS pneumonectomy vs VATS sleeve resections, in the absence of rib spreading.
With proven oncological equivalence to pneumonectomy and favorable rates of operative mortality, overall morbidity, and local recurrence rates, even after induction therapy and for advanced disease, sleeve lobectomy should be adopted as the preferred surgical option for all patients with central NSCLC [19, 20, 34]. A future prospective, randomized controlled trial comparing sleeve lobectomy to pneumonectomy in the management of the central airway tumors is needed. The results of a trial of this nature might be enough to encourage more surgeons to undertake this technically challenging operation in return for improved patient outcomes.
References
Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–54.
Deslauriers J, Tronc F, Gregoire J. History and current status of bronchoplastic surgery for lung cancer. Gen Thorac Cardiovasc Surg. 2009;57:3–9.
Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg. 2008;85:S729–32.
Gonzalez-Rivas D, Delgado M, Fieira E, Fernandez R. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg. 2014;3:E2.
Cusumano G, Marra A, Lococo F, et al. Is sleeve lobectomy comparable in terms of short- and long-term results with pneumonectomy after induction therapy? A multicenter analysis. Ann Thorac Surg. 2014;98:975–83.
Tagawa T, Iwata T, Nakajima T, Suzuki H, Yoshida S, Yoshino I. Evolution of a lung-sparing strategy with sleeve lobectomy and induction therapy for non-small cell lung cancer: 20-Year experience at a single institution. World J Surg. 2016;40:906–12.
Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis. 2016;8:S223–6.
Pan X, Gu C, Wang R, Zhao H, Shi J, Chen H. Initial experience of robotic sleeve resection for lung cancer patients. Ann Thorac Surg. 2016;102:1892–7.
Thomas CP. Conservative resection of the bronchial tree. J R Coll Surg Edinb. 1956;1:169–86.
Allison PR. Course of thoracic surgery in Groningen. Ann R Coll Surg. 1954;25:20–2.
Thomas CP. The present position relating to cancer of the lung. Lobectomy with sleeve resection. Thorax. 1960;15:9–11.
Weisel RD, Cooper JD, Delarue NC, Theman TE, Todd TR, Pearson FG. Sleeve lobectomy for carcinoma of the lung. J Thorac Cardiovasc Surg. 1979;78:839–49.
Jensik RJ, Faber LP, Milloy FJ, Amato JJ. Sleeve lobectomy for carcinoma. A ten-year experience. J Thorac Cardiovasc Surg. 1972;64:400–12.
Gaissert HA, Mathisen DJ, Moncure AC, Hilgenberg AD, Grillo HC, Wain JC. Survival and function after sleeve lobectomy for lung cancer. J Thorac Cardiovasc Surg. 1996;111:948–53.
Pages PB, Mordant P, Renaud S, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg. 2017;153:184–95.
Merritt RE, Mathisen DJ, Wain JC, et al. Long-term results of sleeve lobectomy in the management of non-small cell lung carcinoma and low-grade neoplasms. Ann Thorac Surg. 2009;88:1574–81.
Berry MF, Worni M, Wang X, Harpole DH, D'Amico TA, Onaitis MW. Sleeve lobectomy for nonsmall cell lung cancer with N1 nodal disease does not compromise survival. Ann Thorac Surg. 2014;97:230–5.
Maurizi G, D'Andrilli A, Venuta F, Rendina EA. Bronchial and arterial sleeve resection for centrally-located lung cancers. J Thorac Dis. 2016;8:S872–81.
Maurizi G, D'Andrilli A, Anile M, et al. Sleeve lobectomy compared with pneumonectomy after induction therapy for non-small-cell lung cancer. J Thorac Oncol. 2013;8:637–43.
Gonzalez M, Litzistorf Y, Krueger T, et al. Impact of induction therapy on airway complications after sleeve lobectomy for lung cancer. Ann Thorac Surg. 2013;96:247–52.
Battoo A, Demmy TL, Yendamuri S. Complex thoracoscopic pulmonary resections for the treatment of lung cancer-a review. Indian J Surg Oncol. 2013;4:142–7.
Deslauriers J, Grégoire J, Jacques LF, Piraux M, Guojin L, Lacasse Y. Sleeve lobectomy versus pneumonectomy for lung cancer: a comparative analysis of survival and sites or recurrences. Ann Thorac Surg. 2004;77:1152–6.
Bagan P, Berna P, Brian E, et al. Induction chemotherapy before sleeve lobectomy for lung caner: immediate and long-term results. Ann Thorac Surg. 2009;88:1732–5.
Milman S, Kim AW, Warren WH, et al. The incidence of perioperative anastomotic complications after sleeve lobectomy is not increased after neoadjuvant chemoradiotherapy. Ann Thorac Surg. 2009;88:945–50.
Gomez-Caro A, Boada M, Reguart N, Viñolas N, Casas F, Molins L. Sleeve lobectomy after induction chemoradiotherapy. Eur J Cardiothorac Surg. 2012;41:1052–8.
Park JS, Yang HC, Kim HK, et al. Sleeve lobectomy as an alternative procedure to pneumonectomy for non-small cell lung cancer. J Thorac Oncol. 2010;5:517–20.
Parissis H, Leotsinidis M, Hughes A, McGovern E, Luke D, Young V. Comparative analysis and outcomes of sleeve resection versus pneumonectomy. Asian Cardiovasc Thorac Ann. 2009;17:175–82.
Takeda S, Maeda H, Koma M, et al. Comparison of surgical results after pneumonectomy and sleeve lobectomy for non-small cell lung cancer: trends over time and 20-year institutional experience. Eur J Cardiothorac Surg. 2006;29:276–80.
Ludwig C, Stoelben E, Olschewski M, Hasse J. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg. 2005;79:968–73.
Lausberg HF, Graeter TP, Tscholl D, Wendler O, Schäfers HJ. Bronchovascular versus bronchial sleeve resection for central lung tumors. Ann Thorac Surg. 2005;79:1147–52.
Kim YT, Kang CH, Sung SW, Kim JH. Local control of disease related to lymph node involvement in non-small cell lung cancer after sleeve lobectomy compared with pneumonectomy. Ann Thorac Surg. 2005;79:1153–61.
Bagan P, Berna P, Pereira JC, et al. Sleeve lobectomy versus pneumonectomy: tumor characteristics and comparative analysis of feasibility and results. Ann Thorac Surg. 2005;80:2046–50.
Battoo A, Jahan A, Yang Z, et al. Thoracoscopic pneumonectomy: an 11-year experience. Chest. 2014;146:1300–9.
Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg. 2003;76:1782–8.
Okada M, Yamagishi H, Satake S, et al. Survival related to lymph node involvement in lung cancer after sleeve lobectomy compared with pneumonectomy. J Thorac Cardiovasc Surg. 2000;119:814–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
All authors declare that they have no potential conflicts of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Horan, S., Battoo, A. & Yendamuri, S. Sleeve lobectomy for lung cancer. Indian J Thorac Cardiovasc Surg 34 (Suppl 1), 20–26 (2018). https://doi.org/10.1007/s12055-017-0581-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-017-0581-3