Abstract
Purpose
Pericardial effusion has various underlying etiologies, and clinicians should identify those that require targeted therapy. Pericardial effusion can be drained either with needle aspiration or surgical procedures. In the following article, we opted to report the results of our 8-year experience of surgical pericardial drainage on 235 consecutive patients.
Methods
We retrospectively analyzed the medical records of 235 consecutive patients with pericardial effusion and/or tamponade who were submitted to surgical drainage (subxiphoid or left anterolateral pericardiostomy) between the years 2005 and 2013. We aimed to assess the etiology of pericardial effusion, total intra- and post-procedure drainage, length of in-hospital stay, effectiveness of surgical procedures, and related in-hospital mortality.
Results
We identified 235 patients, 161 (68.51 %) with severe, 63 (26.80 %) with moderate, and 11 (4.68 %) with mild pericardial effusion. Cardiac tamponade was diagnosed in 91 (38.72 %). The most common established etiologies were idiopathic, uremic, and malignant effusion, respectively. Higher total drained volume was more common in pericardial effusions of malignant etiology than idiopathic (mean difference = 272.02, p = 0.005) or iatrogenic (mean difference = 1096.80, p = 0.001). Mean length of post-procedure drainage was 4.6 days. Intra-operative mortality was 0 % and post-operative was 0.8 % (n = 2).
Conclusion
Assessing the data, we concluded that surgical pericardial drainage is a safe and effective method for management of adults with pericardial effusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pericardial effusion (PE) is a relatively common finding in clinical practice. It has various underlying etiologies and clinical presentations. In some instances, presence of PE can be easily related to an associated medical condition or procedure. It may be a consequence of an inflammatory response, malignant or infectious process affecting the pericardium, and obstructed pericardial lymph drainage. However, the exact pathophysiologic mechanism of PE is not well understood in situations such as uremia which there is no obvious inflammation. If accumulation of pericardial fluid is continuing into the pericardial sac, it may cause cardiac tamponade and will be life threatening [1]. PE can be treated medically, be drained by pericardiocentesis or surgical procedures.
The aim of this article is to give a comprehensive review of clinical manifestations, related etiologies, and our surgical experience with patients who underwent surgical pericardiostomy.
Methods and materials
Patients
This study was conducted in the cardiology ward of a general referral teaching hospital. The medical records of 235 patients diagnosed with pericardial effusion who underwent surgical drainage were evaluated in a retrospective observational study through 2005 to 2013.
The inclusion criteria were presence of moderate to severe symptomatic PE, asymptomatic persistent PE, clinically suspected purulent PE, and cardiac tamponade. Patients who refused the surgical pericardiostomy or those with sever comorbidities that made them ineligible for surgery were excluded from the study.
The outcomes of interest included demographic data, clinical presentations, amount of fluid drained intra- and post-operatively, post-operative drainage days, cytology, and biopsy results.
Imaging techniques
Echocardiography
Echocardiograms were obtained using Philips Envicor-C with 2.5–3.5 MHz probe. All echocardiogram examination data were reviewed by an experienced cardiologist.
It was performed on lateral decubitus position. When diastolic echo-free space between the pericardium and left ventricular posterior wall was 20 mm or more, it was classified as large PE, 10–20 mm was determined as moderate, and less than 10 mm was classified as mild. Cardiac tamponade diagnosis was established based on echocardiographic and clinical criteria [2]. Echocardiographic criteria of tamponade were evaluated with two-dimensional (2D) and Doppler echocardiography according to recent guidelines. 2D echocardiographic criteria of tamponade were early diastolic collapse of the right ventricle (RV), late diastolic collapse of the right or left atrium, diastolic right ventricular outlet tract (RVOT) collapse, exaggerated right atrium (RA) collapse during systole, and inferior vena cava plethora.
Doppler criteria of tamponade included exaggerated respiratory variation in right- and left-sided valvular and venous flow, with inspiratory decreases of mitral E flow and increases of tricuspid E flow [3].
Finding the classic symptoms and signs of tamponade such as tachypnea or dyspnea with clear lungs, cool extremities, peripheral cyanosis, diaphoresis, increased systemic venous pressure, hypotension, and pulsus paradoxus, with echocardiographic criteria was definitive of cardiac tamponade.
Chest radiography, as an initial evaluation, was obtained from all 235 patients, and CT scans were performed on 128 through their clinical workup.
Surgical technique
Pericardiostomy was performed under general anesthesia (70.21 %) or local anesthesia with adequate sedation (29.78 %) depending on the patients’ condition and comorbidities. General anesthesia was induced with ketamine. For avoidance of delay in case of probable anesthesia-induced hypotension, the patient’s skin was prepared and draped before induction of anesthesia. For local anesthesia, 2 % lidocaine solution injection was used for patients with major comorbidities such as lung diseases, profound hypotension, and cardiogenic shock secondary to tamponade.
The subxiphoid approach was performed on 29.36 % (n = 69) of the total population who comprised cases with tamponade or suspected purulent or tuberculosis pericardial effusions. In this surgical method, a short midline vertical dermal and subdermal incision was made over the upper abdomen and the xiphoid process and the diaphragm was dissected away retrosternally. The existing layer of fat over the pericardium was removed so that the pericardium could be grasped and pulled down and opened. A piece of pericardium was excised under direct vision, and it was submitted for histopathological examination. All pericardial fluid was decompressed, and it was sent for bacteriologic and cytological analysis. In patients with chronic severe PE, pericardial fluid was drained slowly to prevent acute cardiac dilatation.
The pericardial cavity was examined by finger for detection of any mass or adhesions. Adhesions were gently lysed to enhance drainage. A pericardial catheter was inserted and connected to an underwater seal drainage system. The catheter was removed when the volume of drained fluid became less than 25 ml over 24 h.
Pericardial window via left anterolateral thoracotomy was performed in the remainder of the patients. The procedure consists of an 8–10-cm incision on the fifth or sixth left intercostal space followed by a 4-cm pericardiostomy. The rest of the procedure was performed similar to a subxiphoid approach.
Some of the studied cases (n = 85) such as those who were hemodynamically unstable or those with a persistent PE, had a history of pericardiocentesis before undergoing the surgery. But they were submitted to surgery because of recurrence of PE, previous incomplete drainage, or lack of improvement in their clinical status.
For patients diagnosed with tuberculous pericarditis preoperatively, who had stable hemodynamic status, a four-drug tuberculosis regimen (isoniazid, rifampin, pyrazinamide, and streptomycin) was started before the surgery for a 3-week course. All of them received tuberculosis therapy regimen for 12 months post-operatively. In the sequence of tuberculous PE, surgical drainage was needed if the effusions were persistent after medical treatment with or without periodic needle aspirations with echocardiography guidance, when the pericardium became thickened or when the signs of pericardial constriction were present.
In a month after the hospital discharge, all of the studied patients were followed up with echocardiography and physical examination.
Statistical analysis
All data statistical processing was performed using SPSS version 16.0 for Windows (SPSS, Inc., Chicago, IL, USA). Continuous variables were presented as mean ± standard deviation (SD). The Chi-square test was used to examine the significance of the association between gender and etiology. Normality of the data was checked with Kolmogorov–Smirnov test. When the test statistic would follow a normal distribution, ANOVA and for the non-normal distributions Kruskal–Wallis test were applied. All tests were two tailed, and the p value was considered to be of statistical significance if under or equal to 0.05.
Results
Altogether, medical records of 235 patients were studied. There were 112 males (47.7 %). The mean age was 47.82 ± 15.97 years (ranged from 25 to 81 years). Recorded symptoms and signs are shown in Tables 1 and 2. In our study, the most prevalent symptom was palpitation.
Echocardiographic findings included severe PE in 161 (68.51 %) patients, moderate PE in 63 (26.80 %) patients, and mild PE in 11 (4.68 %) patients. Ninety-one (38.72 %) patients presented with cardiac tamponade which 11, 48, and 61 of them had mild, moderate, and severe PE, respectively. All tamponade subjects with mild PE were hemopericardium which occurred secondary to stab wound (n = 1), coronary angioplasty and percutaneous transvenous mitral commissurotomy (PTMC; n = 3), ablation (n = 1), temporary (n = 3) or permanent pacemaker implantation (n = 1), and aortic dissection (n = 2).
In our study, the causes of PE were idiopathic pericarditis, uremic pericarditis, malignant processes invading the pericardium, tuberculous pericarditis, iatrogenic pericardial effusion, non-tuberculous bacterial pericarditis, collagen vascular pericarditis, dissection (two patients), and trauma (one patient). The etiologic causes of PE and the data on drained pericardial fluid are shown in Table 3.
Malignant processes were diagnosed in 36 (15.31 %) patients. The analysis of pericardial fluid alone, revealed malignancy in 30 of them and failed to diagnose malignant involvement in the remaining six. But histopathological examination confirmed neoplastic involvement of pericardial specimen in all 36 patients. Of the 36 mentioned cases, 16 had lung cancer, 7 had lymphoma, 4 leukemia, 6 breast cancer, 1 esophageal squamous cell carcinoma, 1 gastric adenocarcinoma, and 1 had malignant thymoma.
Tuberculous pericarditis was diagnosed in 21 patients. It was confirmed by analysis of pericardial fluid and tissue. Of a total of 21 patients, 16 (76.19 %) had a positive purified protein derivative (PPD) skin test, 12 (57.14 %) had positive bronchial secretion test for acid-resistant bacilli, and 14 (66.66 %) had chest X-ray findings indicative of TB. Polymerase chain reaction (PCR) analyses of the pericardial fluid or tissue were diagnostic for tuberculosis in 17 (80.95 %) patients, and in the remaining four patients with negative PCR, a positive culture of pericardial fluid was detected. Tubercle bacilli were detected in the smear of the pericardial fluid only in one patient. Pericardial histology showed granulomas in 18 (85.71 %) patients, but the diagnosis of TB was also additionally confirmed by culture.
Non-tuberculous bacterial pericarditis was observed in five patients. The causal microorganisms detected in cultures of pericardial fluid were as follows: Staphylococcus species (n = 3) as a result of drainage of perivalvular abscess into pericardial sac, Pneumococcus species (n = 1) secondary to pneumonia in an immunocompromised patient, and Streptococcus viridans (n = 1).
Collagen vascular pericarditis were detected in five patients who were diagnosed with systemic lupus erythematosus (SLE; n = 3), rheumatoid arthritis (RA; n = 1), and progressive systemic sclerosis (PSS; n = 1).
The mean ± SD total drainage volume was highest in patients with malignancy (1505.56 ± 512.48 ml) and uremia (1420.73 ml ± 429.21). The lowest volume was detected in patients with PE resulting from trauma and dissection (266.66 ml ± 125.83). The average pericardial fluid volume of each group is presented in Table 3.
Statistically relevant differences were observed in relation to the total drained fluid, which was higher in patients with malignant PE than those with idiopathic (mean difference = 272.02, p = 0.005) and iatrogenic (mean difference = 1096.80, p < 0.001) PE.
There were also a statistical relevance between post-operative drainage volume and the etiology of PE in some groups (p value <0.001). For instance, higher amounts were more common in patients with malignant or uremic PE than idiopathic (mean difference = 217.18, p < 0.001) (mean difference = 131.66, p < 0.001), respectively.
The post-operative period for complete drainage (<25 ml/day) was 1 to 8 days (mean, 4.64 ± 1.44 days). Post-operative days in patients with malignant and uremic PE were more than those with idiopathic PE, and it was of statistical significance (p = 0.005 and p = 0.001, respectively).
Patients were hospitalized for 5–15 days (mean, 6 days). Post-operative complications included new-onset atrial fibrillation that was detected in seven patients and five remained persistent, superficial wound infection in nine patients, and need for more than 3 days of ventilator support in nine patients.
Intra-operative mortality was 0 %, but the mortality rate during post-operative hospitalization was two of 235 (0.8 %). One patient died as a result of severe disseminated lung cancer and comorbidities. The other patient was a 91-year-old man with end-stage cirrhosis and severe heart failure. He died of low cardiac output state, despite of pericardial fluid drainage and inotropic support. Follow-up of all patients with echocardiography showed no cases with recurrence of PE after a month.
Discussion
The main issues a cardiologist dealing with PE is faced with are to investigate the underlying etiology and to choose the optimal management. The relative prevalence of various etiologies largely depends on the source of the population studied, the relative size and activity of the different departments in a general hospital (especially the number of patients with neoplastic disease or chronic renal insufficiency who attend each hospital), the study protocol applied, and on the frequency distribution of the different etiologies of pericardial diseases in each geographic area. For instance, in outpatient populations of the western world the most frequent etiologies are probably idiopathic/viral pericarditis and idiopathic pericardial effusion, while in-hospital series neoplastic pericarditis, uremic pericarditis, and iatrogenic disease are prominent etiologies of pericardial effusion. In developing countries, especially in Sub-Saharan Africa, tuberculous pericarditis is the leading cause of pericardial effusion [4].
In our study, which was conducted in a general referral teaching hospital, the most common cause in the population of 235 patients was idiopathic PE. Some studies reported uremic pericarditis as the most prevalent [5, 6]. However, stated by those authors, their studies were conducted single-centered and in hospitals admitting the majority of patients with renal failure for their well-known hemodialysis facilities.
The presentation of PE varies from being asymptomatic to a state of cardiogenic shock secondary to cardiac tamponade. Some patients may experience dyspnea, palpitation, orthopnea, fever, diaphoresis, and chest pain. In our study, palpitation was the most prevalent symptom, but Becit et al. [6] reported a series of 368 cases of PE with dyspnea as the most common symptom.
Cardiac tamponade requires drainage and etiology search. In two other conducted studies, it was reported to be prevalent in 24 to 44 % of patients [7, 8] while it occurred in 38.72 % (n = 91) of our study group.
PE of malignant etiology is a more complex issue. In this study, the term malignant PE was used when either pericardial fluid cytology was positive for malignancy or direct malignant pericardial involvement was detected.
Not necessarily all the pericardial effusions in patients with malignancy are of a neoplastic etiology and other reasons should also be sought. In Jeong’s series [9], malignant pericardial involvement proved by pericardial fluid cytology was reported in 113 (52.6 %) of patients with previous malignancy with dominant cause of ductal cell carcinoma of the breast. In most studies, lung cancer (34–76 %) and breast cancer (10–17 %) are the most prevalent etiology for malignant pericardial effusion [10]. In the current study, the most common cause of malignant pericardial effusion was lung cancer (44.44 %), followed by lymphoma (19.44 %).
Tuberculosis is a relatively common cause of pericardial disease in developing countries, and it is associated with a high mortality rate. Prompt diagnosis of tuberculous pericarditis is difficult. A definitive diagnosis is made by a biopsy specimen or identifying it histologically or isolating the organism from pericardial fluid. Histological examination of pericardial tissue shows organisms or granulomas in 80 to 90 % of patients, but granulomas without Mycobacterium tuberculosis is not diagnostic and can be seen in sarcoidosis and rheumatoid disease [11]. Pericardial histology showed granulomas in 18 (85.71 %) of patients in the current study.
Direct smear examination is very rarely positive, and it ranges from zero to 42 %. Culture of pericardial fluid reveals Mycobacterium tuberculosis in up to two thirds of cases, while pericardial biopsy has a higher yield [12]. PCR is another method which has been used recently, and it can detect Mycobacterium tuberculosis DNA in fluid or tissue of pericardium even in patient with constrictive pericarditis. In our study, PCR analyses were positive in 80.95 % of patients and culture of pericardial fluid confirmed the diagnosis and it was also positive in four patients with negative PCR result.
Bacterial pericarditis may be caused by a variety of organisms. It usually present with a purulent effusion. The most prevalent causal organisms are staphylococci, pneumococci, and streptococci [13]. These were also the same organisms found in the specimens of our patients with bacterial pericarditis, as described previously in the “Results.”
Pericardial involvement can occur in any autoimmune rheumatologic diseases, but it is commonly seen in RA, SLE, and PSS. Acute pericarditis, asymptomatic pericardial effusion, or tamponade may be the initial manifestations [14]. In our study, SLE was the most common cause of PE among patients with connective tissue disorders and no one had tamponade.
Some of complex percutaneous intra-cardiac procedures can also cause cardiac perforation and pericardial effusion. The risk of pericardial fluid accumulation and subsequent tamponade increases with trans-septal puncture and the need for intra-procedural anticoagulation. The best treatment for iatrogenic pericardial effusion varies. In the case of a new small PE without cardiac tamponade, anticoagulation should be reversed and the procedure should be stopped. But in hemodynamically unstable patients, urgent pericardiocentesis is needed. If pericardiocentesis does not improve the patient’s hemodynamics and in the presence of cardiac tear, surgery is required [15]. In our study, all the subjects with hemopericardium (n = 11) failed to respond to pericardiocentesis or performing the procedure was not possible.
Controversies still exist regarding the optimal procedure for management of pericardial effusion and tamponade. A variety of techniques have been utilized. Examples include pericardiocentesis, transcutaneous pericardioscopic pleuropericardial window and subxiphoid pericardial drainage.
Pericardiocentesis has been used for decades and can be of life-saving value in situations like cardiac tamponade. The potential advantages are no need for an incision or general anesthesia and the patient experiences less pain. However, there are still some disadvantages to this technique.
Firstly, it needs to be conducted by an experienced cardiologist especially when inferior locations of the pericardial sac needs decompression. Furthermore, it does not provide visualization and adequate sample of the pericardium. Since some diseases may involve the pericardium, obtaining an adequate pericardial biopsy is not possible with needle aspiration.
Besides, other drawbacks such as incomplete pericardial fluid drainage and a high rate of recurrence comparing with subxiphoid pericardiostomy are reported in the literature [16]. Some studies reported pericardiocentesis a safe method [17, 18], but the study designs excluded patients with multiloculatedor posterior effusions. Even in such settings, we believe that it is true when the cardiologist is an expert. The most dangerous complications of pericardiocentesis are rupture of the myocardium and injury to the coronary arteries, which are reduced nowadays with the employment of echocardiography or fluoroscopy.
Pericardiocentesis should mainly be performed on patients with unstable hemodynamic state or those with a lower risk for recurrence.
Anterior thoracotomy with or without video-assisted thoracic surgery is another method for pericardial fluid drainage. In comparison with subxiphoid drainage, this technique facilitates a well-exposed pericardium for the surgeon to explore, obtain an adequate pericardial biopsy and the PE can be drained completely. The recurrence rate is low with creation of a pericardial window. However, the procedure requires general anesthesia and single-lung ventilation which may be difficult and dangerous in old or critically ill patients [19].
Subxiphoid pericardial window is another method for surgical drainage. This technique allows for adequate biopsy, direct visualization, and exploration of the pericardium and pericardial cavity. Besides, comparing with anterolateral thoracotomy, subxiphoid approach gives the possibility for a rapid intervention which is of vital importance in patients with hemodynamic compromise. However, if the PE is of small amount and localized, this method may be harmful to the heart. But in patients with purulent pericardial effusion, surgical drainage through a subxiphoid route is the procedure of choice [4].
Due to lack of standard management algorithms and on-going controversies regarding the optimal treatment, the choice of procedure is determined in many cases by the cardiologist performing the echocardiogram which depends mainly on the etiology, amount and zone of the PE, clinical situation, and history of the patient.
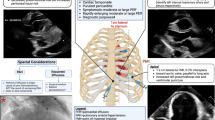
In our experience, there were some indications that made the PE considered eligible to be treated surgically which included PE accumulation in the posterior zone, patient being in severe respiratory distress, presence of clot hemopericardium or numerous fibrin bands, suspected purulent PE, chest deformities that made it impossible to reach the pericardium with a needle, previous unsuccessful pericardiocentesis, or contraindications for needle aspiration. The algorithm we followed in our study was as follows (Fig. 1).
The goal of each procedure should be complete drainage, prevention of recurrence, providing adequate samples for diagnostic studies, and a minimal resultant morbidity and mortality.
For instance, purulent pericardial effusion should usually be drained through a subxiphoid approach. Also, in patients with high recurrence rate of PE such as malignancy or uremia, surgical drainage may be preferred to pericardiocentesis.
In a study conducted by Petcu et al. [20], comparing the results of subxiphoid surgical pericardial drainage and percutaneous catheter drainage in patients with cardiac tamponade, both techniques reported to be safe but re-intervention for recurrence of PE was lower in the surgery group. Reviewing the literature, percutaneous catheter drainage is reported to result in a recurrence rate of 0 to 30 %, with a combined rate of 16.2 %. Open subxiphoid drainage in published reports resulted in a recurrence rate of 0 to 9.1 %, with a combined rate of 3.2 % [17].
Finally, our study was conducted in the cardiology ward of a general hospital with referral settings. We assume that conducting such studies with a multi-centered setting would provide more accurate and subtle results.
To conclude, considering the complete drainage, provision of adequate specimen for diagnostic studies, and low mortality rate, we believe that surgical drainage is an effective approach for diagnosis, treatment, and detection of the underlying etiology of the PE and may be preferred to pericardiocentesis, especially in uremic patients and those with malignancy.
References
Al-Dadah AS, Guthrie TJ, Pasque MK, Moon MR, Ewald GA, Moazami N. Clinical course and predictors of pericardial effusion following cardiac transplantation. Transplant Proc. 2007;39:1589–92.
Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases. Eur Heart J 2004;25:587–610.
Krantz MJ, Lee JK, Spodick DH. Repetitive yawning associated with cardiac tamponade. Am J Cardiol. 2004;94:701–2.
Sagristà-Sauleda J, Mercé AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol. 2011;3:135–143.
Becit N, Özyazicioğlu A, Ceviz M, Karakelleoglu S, Karapolat S, Kocak H. Clinical experience with subxiphoid pericardiostomy in the management of pericardial effusions: a study of 240 cases. J Inter Med Res. 2003;31:312–7.
Becit N, Ünlü Y, Ceviz M, Koçoğullari C, Koçak H, Gürlertop Y. Subxiphoid pericardiostomy in the management of pericardial effusions: case series analysis of 368 patients. Heart. 2005;91:785–90.
Thümmler F, Schmidt H, Evequoz D. Pericardial effusion in the hospital--diagnosis and therapy. . Schweiz Rundsch Med Prax. 1999;88:1573–80.
Yüksel V, Hüseyin S, Okyay A, et al. Management of pericardial effusion by subxiphoidal pericardiostomy in adults. Türk Göğüs Kalp Damar Cerrahisi Dergisi. 2012;20:492–6.
Jeong T-D, Jang S, Park C-J, Chi H-S. Prognostic relevance of pericardial effusion in patients with malignant diseases. Korean J Hematol. 2012;47:237–38.
Kim SH, Kwak MH, Park S, et al. Clinical characteristics of malignant pericardial effusion associated with recurrence and survival. Cancer Res Treat. 2010;42:210–6.
Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s heart disease: a textbook of cardiovascular medicine. 9th ed. Philadelphia: Saunders; 2012.
Trautner BW, Darouiche RO. Tuberculous pericarditis: optimal diagnosis and management. Clin Infect Dis. 2001;33:954–61.
Brook I. Pericarditis caused by anaerobic bacteria. Int J Antimicrob Agents. 2009;33:297–300.
Langley RL, Treadwell EL. Cardiac tamponade and pericardial disorders in connective tissue diseases: case report and literature review. J Natl Med Assoc. 1994;86:149–53.
Holmes DR, Nishimura R, Fountain R, Turi ZG. Iatrogenic pericardial effusion and tamponade in the percutaneous intracardiac intervention era. JACC: Cardiovascular Interventions. 2009;2:705–17.
Allen KB, Faber LP, Warren WH, Shaar CJ. Pericardial effusion: subxiphoid pericardiostomy versus percutaneous catheter drainage. Ann Thorac Surg. 1999;67:437–40.
McDonald JM, Meyers BF, Guthrie TJ, Battafarano RJ, Cooper JD, Patterson GA. Comparison of open subxiphoid pericardial drainage with percutaneous catheter drainage for symptomatic pericardial effusion. Ann Thorac Surg. 2003;76:811–6.
Buchanan CL, Sullivan VV, Lampman R, Kulkarni MG. Pericardiocentesis with extended catheter drainage: an effective therapy. Ann Thorac Surg. 2003;76:817–20.
Muhammad MIA. The pericardial window: is a video-assisted thoracoscopy approach better than a surgical approach? Inter Cardiovasc Thorac Surg. 2011;12:174–8.
Petcu C, Droc I. The Efficiency of Surgical Subxiphoid Pericardial Drainage and Percutaneous Pericardial Drainage in Pericardial Effusions Associated with Cardiac Tamponade. Chirurgia (Bucur). 2013;108:226–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest with respect to the authorship.
Funding
There was no financial support for the investigation.
Rights and permissions
About this article
Cite this article
Azari, A., Manavifar, N., Vakili, V. et al. Surgical pericardial drainage in a series of 235 consecutive patients: an 8-year experience. Indian J Thorac Cardiovasc Surg 32, 250–256 (2016). https://doi.org/10.1007/s12055-016-0461-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-016-0461-2