Abstract
A light-weight multichannel analyser (MCA)-based \(\gamma \)-ray spectrometer, developed earlier at the Inter University Accelerator Centre, New Delhi, has been used as part of the PG curriculum, to determine the effective atomic numbers for \(\gamma \) attenuation of \(^{137}\)Cs \(\gamma \)-ray in different types of samples. The samples used are mixtures of graphite, aluminum and selenium powders in different proportions, commercial and home-made edible powders, fruit and vegetable juices as well as certain allopathic and ayurvedic medications. A narrow beam good geometry set-up has been used in the experiments. The measured attenuation coefficients have been used to extract effective atomic numbers in the samples. The results are consistent with XCOM values wherever available. The present results suggest that the \(\gamma \) attenuation technique can be used as an effective non-destructive method for finding adulteration of food materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Measurement of \(\gamma \)-ray attenuation is useful for studying radiation transport and energy deposition and \(\gamma \)-ray attenuation plays an important role in medical physics, reactor shielding, industrial radiography etc. The concept of effective atomic number [1] is very useful for composite materials in the form of compounds having a definite proportion of the constituent elements as well as mixtures of elements or compounds wherein the corresponding proportions can vary.
The most straightforward way (type I) to obtain the effective atomic number is to fit the theoretical XCOM [2] values for the mass attenuation coefficients with a polynomial as a function of the atomic number Z and to calculate \(Z_{\mathrm {eff,I}}\) using this polynomial at the experimental value of \(\mu \).
Murty [3] derived an empirical relation to calculate the effective atomic number (type II) of heterogeneous materials, consisting of a number of elements in varying proportions as
Here \(w_{i}\) is the weight fraction of the ith element in the composite material, \(Z_{i}\) is the atomic number and \(A_{i}\) is the atomic mass.
Finally, yet another method [4] for extracting effective atomic number (type III) is to first derive the atomic and electronic cross-sections from the measured mass attenuation coefficients as follows:
In the above equations, \(N_{0}\) is the Avogadro number and \(\mu _{i}\) is the mass attenuation coefficient of the ith element.
The effective number is subsequently extracted as the ratio of the above quantities:
Experimental studies aimed at the extraction of effective atomic numbers have been carried out by various investigators in varied types of composite materials such as metallic alloys, building materials like concrete and cement etc. However, such studies have not been done on food materials like edible powders, fruit juices, vegetable extracts etc.
Also, the \(\gamma \)-ray attenuation technique can be used to study adulteration in food items, especially as the method is non-destructive. Chikkappa and Ramesh [5] effectively applied the method to detect melanin contamination in solid milk powder.
In the present study, we have carried out measurements on \(\gamma \)-ray attenuation using \(^{137}\)Cs \(\gamma \)-rays in powder mixtures of the elemental samples of carbon, aluminum, selenium, certain home-made and pure edible powders and their commercial counterparts, some fruit juices and vegetable extracts as well as certain allopathic and ayurvedic medications. From the results thus obtained, we have extracted the effective atomic numbers for \(\gamma \)-ray attenuation using the three methods as described above.
The measurements with the elemental powder absorbers and their mixtures have been carried out for validating the experimental set-up. The studies using edible powders, medicines, juices and vegetable extracts have been undertaken with a view to explore the possibility of applying the technique of \(\gamma \)-ray attenuation for checking adulteration in food materials.
The details of the measurements and the results obtained therefrom are presented below.
2 Experimental details
A \(^{137}\)Cs source procured from BARC, Mumbai was used as the source of 661.6 keV \(\gamma \)-rays for the present study. The \(\gamma \)-rays were attenuated through the selected absorbers in a narrow-beam good geometry set-up with the source and detector aligned coaxially along a vertical axis and allowed to be detected by a well-shielded \(1^{\prime \prime } \times 1^{\prime \prime }\) NaI[Tl] detector coupled to a 1\(^{\prime \prime }\) PMT. This detector forms part of a light-weight MCA-based \(\gamma \) spectrometer, developed earlier at the Inter University Accelerator Centre, New Delhi [6], and used effectively by Kiran et al [7, 8] for the estimation of radioactive \(^{40}\)K content in potassium salts and building materials. The data acquisition and data analysis are carried out using a python code, through a front-end GUI.
All the absorbers were in the form of powders or liquids and are taken in a glass beaker of 11.5 cm\(^{2}\) cross-sectional area, placed on a suitable stand. A sensitive electronic balance was used to determine the thickness of the absorbers in units of g / cm\(^{2}\). The elemental powder absorbers of graphite, aluminum and selenium were obtained from standard companies and have better than 99.5% purity. Some of the edible powders have been procured from commercial sources and some have been home-made. Fruit juices and vegetable extracts have also been obtained from home as well as commercial sources. The allopathic and ayurvedic medications have been supplied by standard pharmaceautical companies.
The measurements have been done using the standard procedure of recording the \(\gamma \)-ray spectra of the incident \(\gamma \)-ray and those transmitted through the various absorbers and extracting the photopeak counts after subtracting the appropriate background counts, determined in a separate run. Comparing the incident and transmitted intensities for each absorber of measured area density, the mass attenuation coefficient is calculated. Thereafter, the corresponding effective atomic numbers are extracted via an interpolation of the theoretical XCOM values.
Typical uncertainties in the quoted attenuation coefficient values have been estimated to be about 2–3%, the major part of which arises from counting statistics and partly due to uncertainties / non-uniformities in the target thickness and mass measurement. The % uncertainties in the extracted effective atomic numbers are similar to that in the attenuation coefficients for type I, whereas those for type III are somewhat larger depending on the slope of the XCOM \(\mu \) plot vs. Z. Type II values have much less uncertainties as these depend only on the mass measurements which have been more accurate.
3 Results and discussion
Table 1 lists the experimental values of the mass attenuation coefficients in the case of the elemental powder absorbers of graphite, aluminum and selenium. For comparison with theory, the corresponding values obtained using the XCOM package [2] are also given.
The experimental results for the mass attenuation coefficients of the mixture absorbers involving pure powder materials of graphite, aluminum and selenium in different proportions are presented in table 2. The effective atomic numbers for the mixtures are extracted from the theoretical XCOM values at different integral values of Z via interpolation.
The present results are seen to be consistent with theoretical XCOM values. Also, for aluminum, our result for the mass attenuation coefficient agrees well with the value of 0.073 obtained by Pravina [9].
We see that the present experimental results for the effective atomic numbers for the mixtures of elemental powders of the three types agree among themselves.
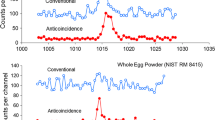
Figure 1 shows a comparison of the spectrum of the direct \(\gamma \)-rays from the source with the spectra of the \(\gamma \)-ray transmitted through the pure maida sample and the packed version, all collected for the same period of time. The spectra of the pure (unprocessed home-made) and packed maida samples apparently overlap on either side of the photopeaks, but definitely show a noticeable difference near the peak, amounting to about 4% difference in the net peak areas. Such a difference in the net photopeak areas translate to about 20% change in the natural logarithms and consequently in the calculated attenuation coefficients.
Table 3 gives mass attenuation coefficients for various edible powders investigated in the present experiment. The corresponding effective atomic numbers are also listed.
In figure 2 a histogram of the above data for the food powders is displayed. A typical error bar is indicated.
Similar results in the case of fruit juices and vegetable extracts are presented respectively in tables 4 and 5. For the fruit juices we have compared the results for the pure juices and the bottled versions.
Figure 3 shows the histogram of the effective atomic numbers for the fruit and vegetable samples.
The results in the case of allopathic syrups and ayurvedic medicines are presented respectively in tables 6 and 7.
4 Discussion and conclusions
It is clear from the values of the mass attenuation coefficients and the effective atomic numbers presented in tables 3–7 that there are notable differences in these values among the various absorbers studied. Comparison of the effective atomic numbers given in figures 2 and 3 show that in most of the cases the deviation between the pure material and the packed versions is beyond the experimental errors. This may be because of the presence of preservatives, added flavours as well as adulterants in them. Thus, a possible criterion for distinguishing these materials based on the mass attenuation coefficients and effective atomic numbers is indicated. Especially in the case of the edible powders, the possibility of using the \(\gamma \) attenuation technique as a non-destructive tool for detecting adulteration is indicated.
The study also points to the possibility of the presence of heavier and possibly polluting elements in bottled juices.
References
P Latha, A M Vinodkumar, K M Varier, B R S Babu, A Joseph, K K Abdullah and M P Unnikrishnan, Rad. Phys. Chem. 81(12), 1817 (2012)
M J Berger, J H Hubbell, S M Seltzer, J S Coursey and D S Zucker, XCOM: Photon cross section database (version 1.2) (Online) (National Institute of Standards and Technology, Gaithersburg, MD, 2003) M J Berger and J H Hubbell, XCOM: Photon cross sections on a personal computer, Program manual (Centre for Radiation Research, National Bureau of Investigations Standards, 1990) M J Berger and J H Hubbell, XCOM version 3.1-NIST Standard Reference Data Base (1999)
R C Murty, Nature 207, 398 (1965)
K Narender, A S Madhusudhan Rao, K Gopal Kishan Rao, N Gopi Krishna and K Ashok Reddy, Res. J. Phys. Sci. 1, 1 (2013)
C Udagani and T N Ramesh, Int. J. Res. App. Nat. Soc. Sci. 2, 81 (2014)
S Venkataramanan and B P Ajith Kumar, Technical report on NaI \(+\) PMT gamma ray spectroscopy system, IUAC\(/\)TR\(/\)SV\(/\)2014-15\(/\)12
K K Kurup, Study of \(^{40}\) K activity in certain potassium salts and building materials, M.Sc. dissertation (University College, Thiruvananthapuram, Kerala, India, 2015)
S Venketaramanan, B P Ajithkumar, K K Kurup and K M Varier, Pramana – J. Phys. 90, 9 (2018)
P P Pawar, J. Chem. Pharm. Res. 3(4), 899 (2011)
Acknowledgements
The authors are thankful to the Director, Inter University Accelerator Centre, New Delhi, for providing the gamma spectrometer system and the associated electronics along with the MCA and the associated software. They are also grateful to the Head, Department of Physics, University College, for providing the necessary facilities for the actual execution of the experimental work described in this paper. One of the authors (KMV) would like to express his thanks to the Kerala State Council for Science, Technology and Environment, Government of Kerala, Thiruvananthapuram for providing an Emeritus Scientist fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Revathy, J.S., Anooja, J., Krishnaveni, R.B. et al. Effective atomic numbers in some food materials and medicines for \(\gamma \)-ray attenuation using \(^{137}\)Cs \(\gamma \)-ray. Pramana - J Phys 90, 72 (2018). https://doi.org/10.1007/s12043-018-1570-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12043-018-1570-9
Keywords
- \(^{137}\)Cs \(\gamma \)-rays
- \(\gamma \)-ray attenuation
- effective atomic numbers
- light-weight multichannel analyser
- mixture samples
- vegetable and fruit juices