Abstract
Pure and Cu-doped zinc oxide (ZnO) nanoparticles were prepared using a chemical method. The dopant concentration (Cu/Zn in atomic percentage (wt%)) is varied from 0 to 3 wt%. Structural characterization of the samples performed using X-ray diffraction (XRD) confirmed that all the nanoparticles of zinc oxide are having polycrystalline nature. Morphological studies were conducted using field emission scanning electron microscopy (FESEM) to confirm the grain size and texture. Electrical measurements showed that the AC conductivity initially decreases and then rises with increasing Cu concentration. The UV–Vis studies showed absorbance peaks in the 200–800 nm region. It is found that the absorbance does not significantly change with doping. This fact is further confirmed from the band-gap calculations using the reflectance graphs. When analysed in terms of Burstein–Moss shift, an increase of band gap from 3.42 to 3.54 eV with increasing Cu concentration is observed. In the Photoluminescence (PL) studies a red-shift is observed with increasing dopant concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metal oxides like zinc oxide (ZnO), tin oxide (SnO2) and indium oxide (In2O3) in their pure form and also in doped forms have been extensively investigated in recent years [1–8]. Among these, ZnO has gained prominence because of its abundance and non-toxicity and also because it is inexpensive compared to others. Zinc oxide (ZnO) is a II–VI semiconductor with a wide band gap of 3.37 eV and a large exciton binding energy of 60 meV at room temperature [9]. Nanoparticles of ZnO are used in a variety of applications: They can be used as UV absorbent, antibacterial treatment material [10], catalytic agent [11,12] and as an additive material in several industrial products. It is currently being used in the fabrication of solar cells [13], gas sensors [14,15], luminescent materials [16], transparent conductors [17], heat mirrors and coatings [18]. Methods such as pulsed laser deposition [19,20], vapour phase transport [21] and chemical vapour deposition [22] have been developed for the preparation of nanostructures of ZnO. Sol–gel method is one of the most important wet chemical methods used for the preparation of metal oxide nanoparticles [23].
Many physical properties of ZnO, such as piezoelectricity, electrical conductivity and defect structures, are greatly influenced by the presence of impurity [24]. Several dopants (Fe, Cr, Al, Cu, Co, Mn, Mg, S, P, etc.) can lead to an increase in the surface area of the ZnO-based powders. The dopants stabilize the ZnO surface, and they also promote changes in the grain size. It was reported that Fe3+-doped ZnO nanoparticles with lower crystallinity and high surface area have higher catalytic activity and sensitivity than that of either the pure ZnO or Fe2O3 [25]. The dielectric properties of pure ZnO are dependent on the presence of interstitial zinc atoms and lack of oxygen at O sites. As pure ZnO is sensitive to oxidation, absorption of O2 results in a decrease of its dielectric properties. Therefore, different dopants have been introduced into ZnO to modify its dielectric and other properties depending on the desired applications. In many cases, when ZnO has been doped, the dielectric properties were reported to be changed because of the extrinsic defects [26].
This study is aimed at the characterization of pure and Cu-doped ZnO nanoparticles synthesized by the chemical method. The dopant concentration of Cu was varied over the range 0 – 3 wt%. Structural and morphological characterizations of the samples were performed using powder X-ray diffraction (XRD) and field emission scanning electron microscopy (FESEM), respectively. Electrical properties were investigated using inductance capacitance resistance (LCR) measurements and optical properties by UV–Visible (UV–Vis) and photoluminescence (PL) spectroscopy. The influence of Cu on the structural, electrical and optical properties is discussed in this paper.
2 Experimental
Zine oxide (ZnO) powder was prepared by the chemical precipitation method and copper chloride (CuCl2⋅H 2O) was used as the source of the dopant. All the reagents were purchased from Merck India Limited and of AR grade. Zinc acetate Zn(CH3COO)2was taken as the precursor and ammonium hydroxide (NH4OH) was taken as the precipitating agent. In this reaction, ammonium acetate provides the hydroxyl ions which plays an important role in capping the size of the particles by excluding the agglomeration of the ZnO particles. The pH of the solution was maintained at 8 by adding suitable amounts of ammonium hydroxide. The following chemical reactions take place:
The resulting precipitate from the reaction was collected and thoroughly washed several times with distilled water and ethanol to remove traces of chlorides. Chlorides were formed because CuCl2·H2O was as the dopant source and the presence of these chlorides severely impaired the functionality of doped ZnO. The as-prepared sample was then dried in an oven for 4 h. The ZnO powder thus obtained was pelletized at a pressure of ∼13 MPa to form cylindrical pellets. The pellets were annealed at ∼850∘C for 12 h in air to improve the crystallinity. Sintered pellets were then polished into discs of 13 mm diameter and 2 mm thickness.
Structural analysis of the pellets was done using PANalyticalX’Pert Pro X-ray Diffractometer with Cu Kα radiation (λ = 0.15418 nm) as X-ray source at 40 kV and 30 mA in the scanning angle (2 𝜃) from 20∘ to 90∘. The samples are also studied using FESEM along with EDS (ZEISS ULTRA 55) to verify the particle size and composition, respectively. Electrical properties (ac conductivity) were measured using the LCR meter Hi-tester (Model:3522-50 Hioki, Japan) over the frequency range 100 Hz – 100 kHz at room temperature with an AC bias of 0.1 mV. For the AC measurements, the flat faces of the pellet were coated with a thin layer of highly conductive silver paste. The pellet was then mounted on a home-made two-probe assembly which was inserted coaxially inside a resistance-heated furnace. UV–Vis studies were performed using CARY 100 Bio UV Visible spectrophotometer (Varian EL 06053308) and PL studies were carried out using Perkin Elmer LS spectrophotometer.
3 Results and discussion
3.1 Structural and morphological studies
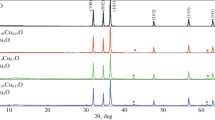
The phase purity and structural parameters are obtained from the powder XRD diffraction patterns. The XRD patterns recorded from undoped and doped pellets with different copper concentration are shown in figure 1. Peaks are found to be quite sharp and intense which implies high crystallinity of samples. The XRD pattern of undoped ZnO is found to match with the ICDD reference pattern (01-070-8072) of ZnO. The analysis of diffraction peaks has revealed the presence of ZnO phase for all the compositions. This indicates that the Cu2+ ions are substituted with Zn2+ ions without any serious effect on the crystalline structure of ZnO. The ionic radius of Cu2+ (0.073 nm) is very close to that of Zn2+ (0.074 nm), due to which Cu can easily penetrate into ZnO crystal lattice.
The crystallite size D is calculated using the Debye–Scherrer formula
where λis the X-ray wavelength, β is the peak FWHM corrected for instrumental broadening and 𝜃 is the angle at which the peak occurs. For undoped ZnO, β= 0.0787, 2 𝜃= 36.3506; for 1wt% Cu doped ZnO, β= 0.096, 2 𝜃= 36.2757 and for 3 wt% Cu-doped ZnO, β= 0.0787 and 2 𝜃= 36.1435, respectively.
The lattice parameters are calculated using Le-Bail and Pawley refinement method. Table 1 shows the unit cell parameters a and c obtained from the XRD data for all the samples. Lattice parameters a and c are found to be slightly greater for Cu-doped samples as compared to those of undoped ZnO. This is due to a slight mismatch in ionic radii between Zn2+ and Cu2+. The possible error range for each parameter is about ±0.5%. However, there is a systematic variation in lattice parameters with increasing Cu content. The c/a parameter has also been found to show a good match with the value for ideally close packed hexagonal structure (1.6333).The volume of the unit cell for hexagonal system has been calculated using the following equation [27]:
The bond length L for Zn–O is given by [28]
The parameter u in the wurtzite structure is given by
The Zn–O bond lengths of undoped and Cu-doped ZnO are given in table 1. It is observed that with the increase in Cu content, bond length increases which is attributed to the segregation of Cu [29,30]. This is visible as some low-intensity peaks in XRD as the dopant concentration increases. This suggests that the crystalline quality of ZnO is improved by Cu doping.
The samples were also studied using FESEM in order to confirm the particle size. Figures 2a–2c show the FESEM micrographs of undoped ZnO, 1 wt% Cu-doped ZnO and 3 wt% Cu-doped ZnO. The figure clearly shows the formation of ZnO nanoparticles for both undoped and doped samples. From the FESEM images it is also seen that there is a wide variation in the particle size though the textures remain more or less consistent. These images also reveal that the particle size decreases with dopant concentration. A thorough analysis of the images was done using image processing software, ‘ZEN’ wherein parameters like average particle size was calculated, which is reported in table 2. It is clearly observed that there is a small deviation in the particle size reported using FESEM data as compared to DS (Debye–Scherrer) or WH (Williamson–Hall) data. However, this is expected as DS calculations do not include changes due to strain whereas WH method is an indirect method where the strain is calculated theoretically. We also observe that similar to DS and WH results, the sizes of the doped ZnO particles obtained by FESEM are smaller than the undoped ZnO particles. The energy dispersive spectroscopy (EDS) results, as shown in figures 2d–2f verify that the samples contain ZnO and Cu only and no other impurities.
A mathematical expression relating the crystallite size and strain-induced broadening was proposed by Williamson and Hall. According to this expression, the peak width as a function of diffraction angle 2 𝜃 is considered for analysing X-ray peak broadening [31,32]. The relation is given as
where β is the FWHM (rad), D is crystallite size (nm), λ is X-ray wavelength and ε is the strain induced on the particles.
Plots shown in figure 3 are drawn with sin 𝜃/λ along the x-axis and β cos 𝜃/λ along the y-axis for high intense peak and, thus, the crystallite size and strain of ZnO −Cu can be obtained from the inverse of the intercept at the y-axis and slope, respectively. The average crystallite size and strain estimated from the Scherrer relation and WH plot are compared in table 2. The difference in crystallite size estimated from Scherrer relation and that from WH plot calculation is mainly attributed to the inclusion of strain in the as-synthesized sample materials and their estimated strain values are reasonable. The possible error range for crystallite sizes is calculated and they are in the range of ±0.55%. Thus, WH plot serves as an additional tool to evaluate and deconvolute crystallite size and strain-induced broadening. The calculated strain is found to be very small in undoped and Cu-doped ZnO samples. This explains why no significant change was observed in XRD pattern. Here, tensile strain is created due to increase in doping concentration and the possible error range estimated is about 0.0050%.
4 Electrical studies
AC conductivity is determined using the relation
where C is the capacitance, ω is the angular frequency and tan δ is the loss factor.
The dependence of σ AC on temperature is given by the relation
where σ 0 is the pre-exponential factor, k is Boltzmann’s constant and E AC is the thermal activation energy.
AC measurements are important means for studying the dynamic properties (conductance, capacitance and dielectric loss tangent) of the semiconducting and dielectric materials. They provide information about the interior of the materials in the region of relatively low conductivity. This measurement also helps to distinguish between localized and free band conduction. In localized conduction, the AC conductivity increases with frequency, while in the free band conduction the conductivity decreases with frequency [33–35]. Further, impedance spectroscopy is a well-known and powerful technique for investigating polycrystalline materials where the contribution of various processes such as bulk conduction, grain boundary conduction and transport across electrode–sample interface can be resolved in the frequency domain [36–39].
The frequency dependence of AC conductivity in undoped ZnO and doped ZnO pellets at room temperature is shown in figure 4. It is seen that the AC conductivity increases slightly with frequency. This type of frequency response indicates localized conduction in the ZnO samples, where AC conductivity increases with frequency.
Higher conductivity is found in pure ZnO and the level decreases with the increase in Cu doping. This may be attributed to the fact, that the-dopant introduces defect ions (such as zinc interstitials and oxygen vacancies) in the ZnO system. These defects tend to segregate at the grain boundaries due to diffusion processes resulting from sintering and cooling. Thus, doping increases the defect concentration and facilitates the formation of grain boundary defect barrier for carriers. This, in turn, decreases the conductivity of the system on doping. However, when the doping level of Cu is high, more Cu ions would get segregated at the grain boundaries. So the density of electrical carriers at the grain boundaries would be high, and the resistance of the grain boundaries would be reduced, thus decreasing the barrier height. In such cases, the doping of Cu has a negative effect on the nonlinear coefficient of the mother sample (ZnO) thereby increasing the conductivity [40,41]. Thus, the AC conductivity is enhanced as the percentage of doping increases from 1 to 3 wt%.
The variations of impedance, tangent loss and capacitance with frequency in pure and Cu-doped ZnO are shown in figure 5. Figure 5a shows that at 1 wt% Cu doping the impedance increases or in other words, the conductivity decreases. However, at higher doping the impedance decreases. This is consistent with the scenario that was observed in case of AC conductivity and which was already explained earlier. Figures 5b and 5c show the variations of tangent loss and capacitance with frequency. From eq. (6), it can be observed that AC conductivity is directly proportional to the tangent loss (tan δ) and also to the capacitance. Consistent with the trend predicted by eq. (6), the variations of the tangent loss and capacitance also show a decrease at 1 wt% Cu doping and then an increase at 3 wt% Cu doping. These changes in impedance, tangent loss and capacitance provide further evidence to support our explanations provided for the results of AC conductivity.
5 Optical studies
5.1 UV–Vis studies
UV–Visible studies are conducted with an intention of measuring the optical band gap of nanocrystals. The transition energy can be easily deduced from the zero crossing of the second derivative of the absorption spectrum. According to the Fermi exclusion principle, for higher photon energies, optical transitions can only occur from the valence band upto the conduction band. The variation of absorbance with wavelength for all the samples is shown in figure 6. It is found that the absorbance initially decreases sharply and then increases slightly. The sharp increase in absorbance at λ < 300 nm is due to interband transitions at the fundamental edge [42]. Band-gap energy increases with decreasing particle size due to quantum size effects. Substitution of Zn2+ by Cu2+ results in an increase in oxygen vacancies and electron concentration due to the electronegativity and ionic radius. This increase in carrier density results in the lifting of the Fermi level towards the conduction band of the degenerate semiconductor and a consequent band-gap widening. A decrease in absorbance at higher doping level of Cu may be attributed to the decrease in the size of nanoparticles and increase in porosity which ultimately widens the band gap [43].
The Kubelka–Munk or remmision function is one of the better methods to determine the reflectance calculations for powder or solid samples. It is found that the absorbance coefficient (α) is proportional to absorption and in turn absorption is proportional to reflectance, F(R). Hence, α is replaced by F(R) in the calculations. The reflectance is given by
The band gap is calculated using the Kubelka–Munk equation [44]. In this equation [ F(R)h ν]2is plotted against h ν(incident photon energy) and the extrapolated line at [ F(R)h ν]2=0 gives the value of band gap in electron volt (eV). The bandgaps obtained are found to be 3.42, 3.50 and 3.54 eV for undoped, 1 wt% Cu doped and 3 wt% Cu doped ZnO, respectively, as shown in figure 7. Thus, the Cu doping increases the band gap of ZnO. Apparently, this change is not very large but it is significant because such small changes have also been reported for Al doping of ZnO, where drastic changes of properties have been observed [45]. The possible error range for the band gap is about ± 0.10%. The error associated with these measured gaps is extracted from the linear regression used to fit the straight lines and from the data points within the straight line region of the data [46].
The change in the optical band gap is explained in terms of Burstein–Moss (BM) band-gap widening. According to the BM effect, the donor electrons in doped semiconductors occupy states at the bottom of the conduction band. In order to conserve linear momentum, the valence electrons require additional energy to be excited to the higher energy states in the conduction band. This is as per Pauli’s principle which prevents states from being doubly occupied and also because optical transitions are vertical. The band-gap shift depends on the carrier concentration in an n-type semiconductor [47].Similar results have been reported for low doping indium in ZnO [48].
5.2 Photoluminescence (PL) studies
Generally, the densities of defects and oxygen vacancies significantly affect the optical properties of oxide nanostructures. The PL spectrum of undoped and Cu-doped ZnO with λ exci= 280 nm is shown in figure 8. The PL spectrum of undoped ZnO exhibits strong near-band-edge emission in the region 445–475 nm. Multiple peaks are observed in the PL spectrum. The origin of these multiple peaks is attributed to carrier transitions from shallow and deep donor levels to acceptor levels of Cu ions (Cu+ and/or Cu2t) in ZnO. The highest intensity peak is due to excitation and the other peaks are due to the defects present in ZnO. These defects increase with Cu doping (as seen from the figure). Cu impurities provide two defect charge states i.e., Cu+ and Cu2tin the sample. The Cu+ defects may be originated by accepting an extra electron from the nearby atom of ZnO and these defects act as shallow acceptors. At higher temperatures, Cu+ changes to Cu2t or Cu2+ thereby produces neutral copper acceptors [49]. It is also observed from the spectra that with the increase in Cu concentration, the UV emission in the Cu-doped ZnO decreases and exhibits a red-shift. As the emission at ∼458 nm is obviously lower than that of the band gap of the ZnO, the visible emission cannot be ascribed to the direct recombination of a conduction electron in the Zn 3d band and a hole in the O 2p valence band [50]. The visible emission is attributed to the electron transition, mediated by defect levels in the bandgap. So, the intensity of the visible emission remains constant as no extra defect level is formed due to the introduction of more Cu atoms into ZnO structure.
6 Conclusion
The structural, electrical and optical properties of sintered ZnO and Cu-doped ZnO nanoparticles prepared by chemical method have been studied. The structural analysis confirms the formation of polycrystalline ZnO in both pure and doped samples. Change in crystallite size is observed with the increasing doping concentration of Cu. Electrical studies confirmed that the conductivity increases slightly with frequency but decreases with doping at lower concentration whereas it increases as the concentration increases. Optical studies indicate interband transitions at the fundamental edge, widening of the band gap and also red-shift with increasing Cu-dopant concentration. The results obtained in the optical studies have been analysed with the help of Kubelka–Munk equation and Burstein–Moss effect. The observations of Cu-doped ZnO reveal that this sample can be chosen as a frontier material in the study of spintronics.
References
A Dev, S Chaudhuri and B N Dev, Bull. Mater. Sci. 31, 551 (2008)
F A Mahmoud and G Kiriakidis, J. Ovonic Res. 5, 15 (2009)
N Kakati, S H Jee, S H Kim, J Y Oh and Y S Yoon, Thin Solid Films 519, 494 (2010)
Ch Y Wang, V Cimalla, Th Kups, C Röhlig, H Romanus, V Lebedev, J Pezoldt, Th Stauden and O Ambacher, J. Appl. Phys. 102, 044310, DOI: 10.1063/1.2770831 (2007)
A Murali, A Barve, V J Leppert, S H Risbud, I M Kennedy and H W H Lee, Nano. Lett. 1, 287 (2001)
G Fan, Y Wang, M Hu, Z Luo and G Li, Meas. Sci. Technol 22, 045203, DOI:10.1088/0957-0233/22/4/045203 (2011)
N Van Hieu, N AnhPhucDuc, T Trung, M A Tuan and N D Chien, Sensors and Actuators B 144, 450 (2010)
A Azam, A S Ahmed, M S Ansari, M Shafeeq and A H Naqvi, J. Alloys Compounds 506, 237 (2010)
T N Soitah, Y Chunhui and S Liang, Sci. Adv. Mater. 2, 534 (2010)
L Sanches, J Peral and X Domenech, Electrochima. Acta 41, 1981 (1996)
W J Huang, G C Fang and C C Wang, Colloids and Surfaces A: Physicochem. Eng. Aspects 260, 45 (2005)
R Annapoorani,MR Dhanjeyan and R Renganathan, J. Photochem. Photobiol. A: Chem. 111, 215 (1997)
K Matsubara, P Fons, K Iwata, A Yamada, K Sakurai, H Tampo and S Niki, Thin Solid Films 431, 369 (2003)
Q Zhang, C Xie, S Zhang, A Wang, B Zhu, L Wang and Z Yang, Sens. Actuators B 110, 370 (2005)
H M Lin, S J Tzeng, P J Hsiau and W L Tsia, Nano Struct. Mater. 10, 465 (1998)
J Zang, W Yu and L Zang, Phys. Lett. A 299, 276 (2002)
S H Keshmiri and M Rezaee Rokn-Abadi, Thin Solid Films 382, 230 (2001)
G Singh, S B Shrivastava, D Jain, S Pandya, T Shripathi and V Ganesan, Bull. Mater. Sci. 33, 581 (2010)
Y Nakata, T Okada and M Maeda, Appl. Surface Sci. 197, 368 (2002)
Y Z Yoo, Z W Jin, T Chikyow, T Fukumura, M Kawasaki and H Koinuma, Appl. Phys. Lett. 81, 3798 (2002)
B J Chen, X W Sun, C X Xu and B K Tay, Physica E 21, 103 (2004)
Y J Li, R Duan, P B Shi and G G Qin, J. Cryst. Growth 260, 309 (2004)
W L Dai, Y Cao, L P Ren, X L Yang, J H Xu, H X Li, H Y He and K N Fan, J. Catal. 229, 80 (2004)
X S Wang, Z C Wu, J F Webb and Z G Liu., Appl. Phys. A: Mater. Sci. Process. 77, 561 (2003)
L M Fang, X T Zu, Z J Li, S Zhu, C M Liu, W L Zhou and L M Wang, J. Alloys Compd. 454, 261 (2008)
W H Zhang, W D Zhang and J F Zhou, J. Mater. Sci. 45, 209 (2010)
H Abdullah, M N Norazia, S Shaari, M Z Nuawi and N S Mohamed Dan, Am. J. Eng. Appl. Sci. 3, 171 (2010)
B D Cullity, Elements of X-ray diffractions(Addison-Wesley, Reading, MA, 1978)
G Srinivasan, R T R Kumar and J Kumar, J. Sol-Gel Sci. Technol. 43, 171 (2007)
S Singhal, J Kaur. T Namgyal and R Sharma, Physica B 407, 1223 (2012)
I Khan, S Khan, R Nongjai, H Ahmed and W Khan, Opt. Mater. (2013) (in press)
V Biju, S Neena, V Vrinda and S L Salini, J. Mater. Sci. 43, 1172 (2008)
R Ondo-Ndong, G Ferblantier, F Pascal-Delannoy, A Boyer and A Foucaran, Microelectron. J. 34, 1087 (2003)
D J Nagaraju and S B Krupanidhi, Mater. Sci. Eng. B 133, 70 (2006)
P P Sahay, S Tewari and R K Nath, Cryst. Res. Technol. 42, 275 (2007)
Y W Hong and J H Kim, Ceram. Int. 30, 1307 (2004)
M Andres-Verges and A R West,J. Electroceram. 1, 125 (1997)
J Jose and M A Khadar, Nanostruct. Mater. 11, 1091 (1999)
E J Abram, D C Sinclair and A R West, J. Electroceram. 10, 165 (2003)
N R Yogamalar, R Srinivasan, A Vinu, K Ariga and A Chandra Bose, Solid State Commun. 149, 1919 (2009)
Z Penga, X Fub, Y Zanga, Z Fua, C Wanga, L Qic and H Miao, J. Alloys Compounds 508, 494 (2010)
J F Wang, W B Su, H C Chen, W X Wang and G Z Zang, J. Am. Ceram. Soc. 88, 331 (2005)
S Tewari and A Bhattacharjee, Pramana – J. Phys. 76, 153 (2011)
D Bao, X Yao, N Wakiya, K Shinozaki and N Mizutani, Appl. Phys. Lett. 79, 3767 (2001)
P Chetri and A Choudhury, Physica E 47, 257 (2013)
S C Das, R J Green, J Podder, T Z Regier, G S Chang and A Moewes, J. Phys. Chem. C 117, 12745 (2013)
A Alaeddine, I Rachidi, F Bahsoun, Y Mohanna, O Bazzi and F El Haj Hassan, J. Appl. Sci. 9, 1588 (2009)
G Singh, S B Shrivastava, D Jain, Swati Pandya, T Shripathi and V Ganesan, Bull. Mater. Sci. 33, 581 (2010)
K Das, S Ray, S Chaudhuri and A B Maity, Indian J. Pure Appl. Phys. 47, 377 (2009)
E Burstein, Phys. Rev. 93, 632 (1954)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
GHOSH, A., KUMARI, N. & BHATTACHARJEE, A. Influence of Cu doping on the structural, electrical and optical properties of ZnO. Pramana - J Phys 84, 621–635 (2015). https://doi.org/10.1007/s12043-014-0851-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12043-014-0851-1