Abstract
Cowpea [Vigna unguiculata L. (Walp)] is grown mainly for its protein-rich grains and is consumed in various forms in sub-Saharan Africa. Average grain yield in farmers’ fields is generally low due to a number of biotic and abiotic stresses. One hundred and six cowpea accessions from Ghana, which had previously been evaluated for seedling drought tolerance, were used for this study. This paper attempts to use three multi-locus PCR-based molecular markers; simple sequence repeats (SSR), inter-retrotransposon amplified polymorphism (IRAP) and retrotransposon-microsatellite amplified polymorphisms (REMAP), to analyse genetic diversity in the cowpea accessions. Analysis of the polymorphic bands data indicated that 101 alleles were amplified among 121 cowpea genotypes (83.4%) from 16 SSR primer pairs out of a total of 30 SSR primer pairs. Likewisely, a total of 66 (54.5%) polymorphic bands were obtained from IRAP and a total of 114 (94.2%) highly polymorphic bands obtained from REMAP analysis. The outcome indicated the highly polymorphic nature of the DNA markers, as small groups of these molecular markers were found to be able to identify each of the accessions used. Microsatellite markers (SSRs) and retrotransposon-based markers, like IRAP and REMAP, were found to be highly polymorphic and informative, suggesting that genomic fingerprinting has a major role in characterizing populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cowpea [Vigna unguiculata (L) Walp] is an important staple food crop in Ghana and many other parts of the world (Obembe 2008; Timko and Singh 2008). The crop provides strong support to the livelihood of small-scale farmers through its contributions to their nutritional security, income generation and soil fertility enhancement. Worldwide about 6.5 million metric tons of cowpea are produced annually on about 14.5 million hectares of land (Boukar et al. 2016). The cowpea grains contain on average 25% protein, 53.2 mg/kg iron, 38.1 mg/kg zinc, 826 mg/kg calcium, 1915 mg/kg magnesium, 14,890 mg/kg potassium, and 5055 mg/kg phosphorus (Boukar et al. 2011). The low productivity of cowpea is attributable to numerous abiotic and biotic constraints. The abiotic stress factors comprise drought, low soil fertility and heat, while biotic constraints include insects, diseases, parasitic weeds, and nematodes (Asare et al. 2010). Cowpea is generally considered to be a self-pollinating crop, which makes its genetic base narrow (Sharawy and El-Fiky 2003; Poehlman 2013).
Genetic diversity is known to provide the fundamental insight for biological diversity and selective breeding studies (Glaszmann et al. 2010; Kouam et al. 2012). Limited genetic diversity poses a threat to the survival of species as it limits their ability to respond to changes in climate, pathogen populations and agricultural practices (Manifesto et al. 2001). The source of genetic resources for crop improvement is the available germplasm in genebanks and this needs to be assessed for availability of useful traits for crop improvement (Tan et al. 2012). Cowpea is one of the most researched crops at the genebank of the Council for Scientific and Industrial Research – Plant Genetic Resources Research Institute (CSIR – PGRRI) in Ghana (Egbadzor et al. 2014). These cowpea accessions were collected from different geographical areas of Ghana about three decades ago and were mostly characterized based on morphological (Bennett-Lartey 1992), seed protein (Oppong-Konadu et al. 2005), and drought tolerance traits (Otwe et al. 2011).
Knowledge of genetic distance and diversity at the molecular level among germplasm is important for characterization and identification of gene flow among populations. While numerous studies have evaluated molecular diversity of common bean (Biswas et al. 2010; Zargar et al. 2014; Zargar et al. 2016), a number of reports related to cowpea genetics have focused on linkage maps and genome analysis (Sharp et al. 2000; Gale et al. 2001; Menz et al. 2002). Information on molecular diversity of cowpea is still limited and data on molecular variation at the DNA level of Ghanaian cowpea is lacking. However, simple sequence repeat (SSR) markers have been used to evaluate genetic diversity and phylogenetic relationships of cowpea genotypes (Asare et al. 2010; Badiane et al. 2012). It is critical, for the purposes of efficiency, that the best available tool for genetic diversity assessment is deployed.
Currently, the use of molecular markers for predicting hybrid performance, gene discovery, genetic diversity, molecular breeding and population genetics has become the method of choice and has revolutionized molecular analysis (Abdollahi Mandoulakani et al. 2015). A major step forward in genetic identification is the discovery that about 30–90% of the genome of virtually all the species is constituted by the regions of repetitive DNA, which can be highly polymorphic in nature (Kalendar et al. 2011). Molecular markers have many advantages over phenotypic characters as they are unaffected by the environment.
DNA-based markers, such as simple sequence repeats (SSRs), have been and are still being utilized in cultivar development, quality control of seed production, measurement of genetic diversity for conservation management, varietal identification and intellectual property protection (Smith et al. 1997). The simplicity of the banding pattern and the multi-allelic nature of SSR loci may be extremely useful in interpreting segregation data (Badiane et al. 2012). Inter-retrotransposon amplified polymorphism (IRAP) and Retrotransposon microsatellite amplified polymorphism (REMAP) are potential markers due to the abundance of retrotransposons (RTNs) in eukaryotic genomes and their ability to create new copies (Abdollahi Mandoulakani et al. 2015). IRAP uses PCR primers designed as an outward direction from the conserved sequences of long terminal repeats (LTR). REMAP, on the other hand, resembles IRAP but uses one LTR primer and a primer specific to a nearby microsatellite. The major challenge with the use of IRAP and REMAP markers is that sequence of the RTNs is required for primer design (Kalendar 2011). It is extremely important to study the genetic composition of the germplasm using molecular markers in order to enhance the understanding of the genetic variations among the existing cultivars, for effective planning of crosses and breeding for the trait of interest. Therefore, this paper examines the diversity of an extensive range of cowpea germplasm sampled from Ghana. Diversity analysis using three multi-locus PCR-based molecular markers (SSR, IRAP and REMAP) were performed. The objective was to establish the pattern of diversity, discriminating capacity and the effectiveness of SSR, IRAP and REMAP marker techniques in the cowpea germplasm used.
Results
The SSR, IRAP and REMAP reactions amplified DNA sequences from cowpea accessions with high reliability. Many primer pairs identified extensive polymorphisms between the accessions analysed. Table 1 shows the details of the 16 primers used for the SSR analysis. Analysis of the data generated from the scoring of the polymorphic bands indicated that 101 alleles were amplified among 121 cowpea genotypes (83.4%) from 16 SSR primer pairs.
Patterns of Diversity of the SSR, IRAP and REMAP Markers
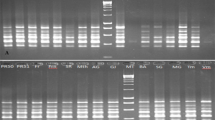
The level of polymorphisms exhibited by the SSR markers was revealed through the number of amplified alleles per primer pair. They varied from 4 for VM 26, 37 and 38 to 11 for VM 28, with the mean number of alleles being 6.31 (Table 1). The allele frequencies for all the primers were generally below 0.95 indicating that they were all polymorphic in character. Gene diversity was high, ranging from 0.59 in VM 19 to 0.77 in VM 28 (Table 1). The sizes of amplified alleles ranged from 110 to 310 bp depending upon their respective expected product sizes as shown in Table 1 and Fig. 1 (a and b). The 16 SSR markers were able to produce polymorphic bands in most of the 106 accessions from Ghana and 15 others from Nigeria and the United Kingdom used for the study. Indeed, the three most polymorphic loci were VM17, VM35, and VM36 with clear polymorphic patterns. The IRAP analysis also produced an appreciable level of polymorphisms (54.5%). All the six IRAP primer combinations (Table 2) generated multiple fragments of defined sizes from the genomic DNA of all the cowpea accessions (Fig. 2 a–d). On average, single Cicer and LTR primers yielded 8 to 14 polymorphic bands, with the highest and the smallest number of bands obtained with the Cicer/Cicer combinations (14 bands) and 3′ LTR/3′ LTR (8 bands), respectively (Table 3). The product sizes ranged from 100 bp to about 2.5 kbp as shown in Fig. 2 (a–d). The REMAP analysis was performed with 8 primer combinations, generating multiple fragments of defined sizes from the genomic DNA of all cowpea accessions. A total of 114 (94.2%) highly polymorphic bands were obtained (Table 3) which were reproducible. The primer combinations that amplified the highest and lowest number of bands were Cicer/BT-CTG (18 bands) and TY-2R/BT-CTG (10 bands), respectively. The product sizes ranged from 100 bp to about 3 kbp as shown in Fig. 3 (a–d).
Polymorphism patterns based on primer combination (a–d). a Primer combination Cicer + BT-GAC; b Primer combination Cicer + BT-CTG; c Primer combination TY-2R + BT- GAC; d Primer combination TY-2R + BT-CTG. The arrowed represents the unique bands identified for future analysis. L = marker; 1, 2, 3, ……16 represent cowpea lines
Discriminating Capacity of the SSR, IRAP and REMAP Markers
The polymorphic information content (PIC) calculated helped to assess the discriminating power of each of the markers used in the study. The PIC of SSR markers ranged from 0.53 for VM 19 and 40 to 0.74 for VM 28, with an average of 0.60. PIC values positively correlated (r = 0.65) with the number of amplified alleles per primer. The observed heterozygosity (H0) calculated for each SSR locus ranged from 0.03 to 0.14 with the mean being 0.08 (Table 1). The lowest H0 value was recorded for VM 40 while the highest value was for VM 36. These low observed heterozygosity values were significant since they tend to substantiate the homozygous nature of most of the accessions and the fact that cowpea is largely self-pollinated.
Effectiveness of SSR, IRAP and REMAP Markers
The phylogenetic tree generated from the scores of SSR markers and calculated genetic distances (Fig. 4), generally, agreed with the nature of the lines used for the study. The cowpea varieties acquired from Leicester, the United Kingdom, clustered as an out-group together with other accessions from IITA, Nigeria. Among the 61 accessions used to construct the phylogenetic tree, there were no strong significant groupings, indicating that the diversity represented by these SSR marker alleles was widely distributed both geographically and across the taxa. However, broad groupings, mostly with similarities in both locality and taxon, were evident in the trees. The phylogenetic tree generated from the scores of IRAP (Fig. 5) markers also showed the same pattern as shown by the SSR tree by separating the Leicester cowpea out-group from the accessions from Ghana and Nigeria, generally. Thus, the results obtained reflected the trend and effectiveness of the three markers used for the study.
Phylogenetic relationship among 61 cowpea lines revealed by cluster analysis (UPGMA) based on genetic similarity (Nei et al. 1998) using 16 microsatellite polymorphic primers.  = Drought Tolerant;
= Drought Tolerant;  = Drought Susceptible;
= Drought Susceptible;  = Leicester Out-group;
= Leicester Out-group;  = IITA
= IITA
Discussion
Knowledge of genetic variation has important implications for the conservation of genetic resources and breeding programs. The relative genetic diversity can be estimated using various approaches including pedigree information, morphological and molecular markers. Morphological markers are influenced by prevailing environmental conditions (Otwe et al. 2011). DNA-based markers offer consistent results regardless of cropping conditions, type, and age of sampled tissue (Schulman et al. 2012). These characteristics of DNA-based markers make them suitable for cowpea research. In the present study the genetic relationships between 121 cowpea lines using multi-locus DNA markers, SSR, IRAP and REMAP has been determined.
Considering the patterns of diversity of the markers used for this study, the results showed that SSR markers were generally highly polymorphic. Microsatellite markers have shown a high level of polymorphism in many important crops, including rice (Islam et al. 2012; Sajib et al. 2012; Singh et al. 2016), maize (Molin et al. 2013; Salami et al. 2016), sorghum (Galyuon et al. 2016), soybean (Chauhan et al. 2015), common bean (Zargar et al. 2016), and tomato (Zhou et al. 2015). The SSR markers could distinguish cowpea accessions used in the study to a large extent. Twenty-two out of the thirty microsatellite primer pairs used in the study could successfully amplify DNA from the cowpea accessions (73.3%), and sixteen of these primer pairs were polymorphic (72.7%). SSR primers detected 4 to 13 alleles among 48 wild lines of cowpeas with an average of 7.5 alleles per primer (Li et al. 2001). Similarly, sixteen SSR primers generated a range of 5 to 12 allele fragments with an average of 8.2 alleles per primer combination among cowpea genotypes (Sawadogo et al. 2010). The results from our study were in agreement with these recent reports as the number of alleles ranged from 4 to 11 with an average number of alleles being 6.3 (Table 1). Similarly, 25 informative SSR primers were used to analyze Ghanaian cowpea germplasm and it yielded 1 to 6 alleles per primer pair with a mean of 3.8 alleles (Asare et al. 2010). The allele frequency for the 16 primers used for the study ranged from 0.34 to 0.57 with a mean frequency of 0.46. This also compares with the average allele frequency reported by Desalegne et al. (2016) of 0.47 and Doumbia et al. (2014) whose allele frequency ranged from 0.15 to 0.45 with a mean of 0.28. Thus, the level of microsatellite polymorphism in cowpea, although relatively high, is much lower than in other crops (Desalegne et al. 2016). One possible reason could be that the materials used in the study were mostly from the Ghanaian open market or directly from farmers and, thus, had a relatively narrow genetic base (Kuruma et al. 2008; Doumbia et al. 2014). Another possible reason for the low level of microsatellite polymorphism is that the cultivated cowpea is relatively low in genetic diversity compared with other crops (Xiong et al. 2016).
The results of our study also supported the fact that IRAP and REMAP techniques had the ability to detect high levels of polymorphism. The degree of polymorphism of the IRAP and REMAP products in cowpea was high (Table 3) which were similar to those observed in barley (Kalendar et al. 2011), olive (Ergun and Yilmaz-Gokdogan 2016) and rice (Yuzbasioglu et al. 2016). Retrotransposons can potentially integrate into either orientation, enabling the finding of members of a retrotransposon family as head-to-head, head-to-tail and tail-to-tail (Schulman 2007). As indicated in Table 3, all the primer combinations for IRAP gave quite a high degree of polymorphism with the least from the 3′ LTR/3′ LTR combination whose orientation was tail-to-tail. This may probably suggest that the integration level of copia-retrotransposons in tail-to-tail orientation in cowpea is lower than the other two orientations (head-to-tail and head-to-head), but needs further analysis using other combinations of primers.
Our results also gave some insight into the discriminatory potential of the three-multi locus markers used. Polymorphic information content (PIC) measures the discriminatory ability of a locus. Data reported by Kuruma et al. (2008) showed polymorphic information content (PIC) ranging between 0.09 and 0.87 with a mean of 0.34. The mean PIC value (0.60) of the SSR recorded in this study compared favourably with results obtained by Sajib et al. (2012), of mean PIC of 0.48 in some rice genotypes. The observed heterozygosity (H0) calculated for the 16 SSR loci for our study also ranged from 0.03 to 0.14 with the mean of 0.08. The cowpea crop is known to be generally a self-pollinating plant, which these results seem to ascertain. It also probably indicates that the accessions used could mostly be homozygous in nature. Genetic diversity of cultivated cowpea and its wild species have been extensively investigated in legume crops (Badiane et al. 2012; Abdollahi Mandoulakani et al. 2015). It has been suggested that cowpea was only domesticated once (Asare et al. 2010), unlike P. vulgaris (Singh et al. 2016) or rice (Sweeney and McCOUCH 2007). The low genetic diversity in cultivated cowpea, therefore, may be attributed again to its narrow genetic base. Studies in Azuki bean had demonstrated that genetic diversity was low and less within the cultigens as compared to their wild relatives, where the genetic diversity was high (Xu et al. 2008). The current SSR study has demonstrated that microsatellite markers might be conserved among Vigna species; hence, could provide a simple approach to assaying the introduction of such genetic material.
The results of the cluster analysis of cowpea accessions in both the SSR and IRAP techniques (Figs. 2 and 5) were indicative of the fact that the markers selected for the study were efficient in identifying the genetic variability within the collection. In the microsatellite phylogenetic tree (Fig. 2), sixteen Ghanaian accessions were observed to have clustered below a branch point at the lower part of the tree and all these accessions have been determined previously to be drought-tolerant (Otwe et al. 2011). Above this group of clusters were 11 drought-susceptible and 18 drought-tolerant Ghanaian accessions. Although the bootstrap values of these branch points were low and not conventionally significant, the results of the cluster analysis were notable in showing that a group of accessions defined by several branch points was all drought-tolerant, while other accessions included a mixture of susceptible and tolerant genotypes. Within the top half of the tree, both tolerant and susceptible lines were observed, but there was little structure evident as some of the most closely related pairs of accessions included both susceptible and drought-tolerant lines. Similarly, the phylogenetic model of the IRAP data, based on six primer combinations for 16 cowpea accessions (Fig. 5), showed interesting clustering results, indicating very low genetic distances between the groups and non-significant bootstrap values. Both the SSR phylogenetic and IRAP trees could cluster most of the Leicester lines as the out-group and, therefore, were different from the African lines. However, the Leicester lines in both situations did not represent much diversity. It is obvious from our study and as reported by other researchers (Biswas et al. 2010; Abdollahi Mandoulakani et al. 2015) that measured relative genetic distances among the studied lines, as well as the techniques used, failed to correlate with the source and drought tolerant nature of the lines. The results obtained with the use of IRAP and REMAP techniques have proven to be generally reliable molecular markers with great potential to be used in genome assessments for fingerprinting, mapping and diversity studies. Therefore, multi-locus markers of cowpea could be used in germplasm conservation and analysis, not only for breeding lines and cultivars but also for the wild cowpea species and other Vigna species. In addition, these multi-locus markers could be used for comparative genome analysis between the different Vigna species.
Conclusion
In this paper we have presented data to support a brief glimpse into the ability of SSR, IRAP and REMAP marker techniques to establish the pattern of diversity, their discriminating capacity and their general effectiveness in determining structure. We could not establish significant associations between the molecular markers, though the results obtained have established a substantial pattern of diversity, some discriminating capacity and the general effectiveness of SSR, IRAP and REMAP marker techniques in determining molecular diversity in the Ghanaian cowpea. We believe that this type of research has contributed in shaping our focus on diversity studies in cowpea. Work is in progress to increase the element specific primer combinations, which have been designed from cowpea genome and inter-primer binding sequences (iPBS). The multi-locus PCR-based markers have potential to be an effective tool for diversity analysis in cowpea, which may be useful in identifying promising candidates for interspecific hybridization programmes and marker-assisted selection (MAS).
Materials and Methods
Plant Material
Plant material consisted 106 cowpea [Vigna unguiculata L. (Walp)] genotypes collected from all agro-ecological regions of Ghana, 10 genotypes from Nigeria and 5 genotypes from the United Kingdom were used for the study. For the molecular diversity analysis, fresh young leaves of 20-day-old seedlings were harvested, wrapped in aluminium foil and flash-frozen in liquid nitrogen and the genomic DNA extracted from these young leaves following a modified cetyltrimethylammonium bromide (CTAB) method (Gale et al. 2001). The quality and the concentration of the DNA were determined using a spectrophotometer and electrophoresis in a 2.0% (w/v) agarose gel.
SSR Primers and Reactions
Thirty SSR primer pairs that previously showed clear polymorphisms in cowpea (Li et al. 2001) were used in this study. SSR assays were performed according to (Colebatch et al. 2002) with minor modifications. The reactions were performed using a Touchdown amplification procedure, depending upon the annealing temperature™ of the primers used. The general amplification cycle consisted of 18 cycles of 94 °C for 1 min (denaturing) and 72 °C for 1 min (extension). The annealing temperatures (30 s) were progressively decreased by 0.5 °C every cycle from 64 to 55 °C. The PCR reaction then continued for 30 additional cycles at 94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1 min. The reaction ended with a 10-min extension at 72 °C. Amplification products were initially separated on 2% agarose gel in 1× TAE buffer stained with 0.5 μg/ml ethidium bromide and those found to be polymorphic were subsequently separated on 6% denaturing Polyacrylamide gels, and the oligonucleotide products visualized by silver-staining.
IRAP Primers and Reactions
The IRAP amplification reaction was performed according to the protocol described by (Kalendar et al. 1999). The primer sequences, retrotransposon source, and orientation are shown in Table 2. An additional degenerate IRAP primer, designed from a multiple sequence alignment of chickpea (Cicer) retrotransposon sources, was also used and is shown in Table 2. The inter-retroelement amplified polymorphism (IRAP) PCR was performed in a 25 μl reaction mixture containing 50 ng DNA, 10X PCR buffer (Promega cat. No. M1861), 2 mM MgCl2, 5 pmol of each primer, 200 μM dNTP mix, 1 U Taq polymerase (Promega, cat. No.1861). The PCR programme consisted of 95 °C for 2 min for initial denaturation followed by 30 cycles of 95 °C for 1 min, annealing at a specified Ta depending on the specific primer combination used for 1 min, extension at 72 °C for 2 min and a final extension at 72 °C for 10 min. The PCR products were then electrophoresed on 3% agarose gel (w/v) and the bands detected by 0.5 μg/ml ethidium bromide staining.
REMAP Primers and Reactions
The primers used for the IRAP amplification were combined with SSR primers (BT-GAC, BT-CTG, and Musa Ty2R) producing eight LTR-SSR primer combinations, as shown in Table 2, in the REMAP experiments. REMAP amplifications were performed in a final volume of 25 μl, containing 50 ng DNA, 10× PCR buffer (Bioline/York Bio), 2.5 mM Mgcl2, 0.25 μM dNTPs (Bioline), 0.4 μM of each primer and 0.5 U of Taq polymerase (Bioline/York Bio) in a T-Gradient Thermocycler (Biometra, Goettingen) 96-well plate. The amplification programme consisted of an initial denaturation cycle at 94 °C for 2 min, followed by 30 cycles at 94 °C for 30 s, 52 °C for 2 min, and 72 °C for 2 min for denaturation, annealing, and extension, respectively. A final extension step was performed at 72 °C for 10 min. The amplification products were separated on 3% agarose gel stained with ethidium bromide.
SSR Data Scoring and Analysis
DNA fragments for the SSR markers were scored visually both from the agarose gel as well as from the scanned images. For each gel, the distance travelled by each marker size of the DNA ladder was measured using a ruler in Adobe Photoshop Elements 2.0. The PowerMarker software package version 3.25 (Liu and Muse 2005) was used to analyse the data obtained and to calculate similarity coefficients among the genotypes. For clarity purposes, a phylogenetic tree was constructed from the similarity coefficient distance matrix of 61 randomly selected cowpea lines using the UPGMA method, instead of the 121 cowpea lines. To investigate the discriminatory power of each SSR primer, the polymorphic information content (PIC) was calculated. The observed heterozygosity (H0) for each primer set was also obtained.
IRAP and REMAP Data Scoring and Analysis
The amplified fragments for both IRAP and REMAP were scored independently as 1 and 0 for presence and absence at each position, respectively, and the obtained binary data were used for the analysis. The genetic similarity between individual pairs of genotypes was analysed by using the MEGA version 4 (Tamura et al. 2004). The average similarity for all genotype pairs was used as a cut-off value for defining the clusters. The statistical stability of the clusters was also estimated by a bootstrap analysis with 1000 replications, using the MEGA version 4 (Tamura et al. 2004).
Abbreviations
- IRAP:
-
Inter-retrotransposon amplified polymorphism and
- REMAP:
-
Retrotransposon-microsatellite amplified polymorphisms
- SSR:
-
Simple Sequence Repeats
References
Abdollahi Mandoulakani B, Sadigh P, Azizi H, Piri Y, Nasri S, Arzhangh S (2015) Comparative assessment of IRAP, REMAP, ISSR, and SSR markers for evaluation of genetic diversity of alfalfa (Medicago sativa L.) J Agric Sci Technol 17:999–1010
Asare AT, Gowda BS, Galyuon IK, Aboagye LL, Takrama JF, Timko MP (2010) Assessment of the genetic diversity in cowpea (Vigna unguiculata L. Walp.) germplasm from Ghana using simple sequence repeat markers. Plant Genet Resour 8:142–150
Badiane F, Gowda B, Cissé N, Diouf D, Sadio O, Timko M (2012) Genetic relationship of cowpea (Vigna unguiculata) varieties from Senegal based on SSR markers. Genet Mol Res 11:292–304
Bennett-Lartey S (1992) Plant genetic resources activities in Ghana. A status report. In: Proceedings of the workshop on Bioversity. University of Ghana, Legon, Accra, 8th January
Biswas M, Hassan J, Hossain M (2010) Assessment of genetic diversity in French bean (Phaseolus vulgaris L) based on RAPD marker. Afr J Biotechnol 9:5073–5077
Boukar O, Massawe F, Muranaka S, Franco J, Maziya-Dixon B, Singh B, Fatokun C (2011) Evaluation of cowpea germplasm lines for protein and mineral concentrations in grains. Plant Genet Resour 9:515–522
Boukar O, Fatokun CA, Huynh B-L, Roberts PA, Close TJ (2016) Genomic tools in cowpea breeding programs: status and perspectives. Front Plant Sci 7:757. doi:10.3389/fpls.2016.00757
Chauhan DK, Bhat J, Thakur A, Kumari S, Hussain Z, Satyawathi C (2015) Molecular characterization and genetic diversity assessment in soybean [Glycine max (L.) Merr.] varieties using SSR markers. Indian J Biotechnol 14:504–510
Colebatch G, Cooper P, East P (2002) cDNA cloning of a salivary chymotrypsin-like protease and the identification of six additional cDNAs encoding putative digestive proteases from the green mirid, Creontiades Dilutus (Hemiptera: Miridae). Insect Biochem Mol Biol 32:1065–1075
Desalegne BA, Mohammed S, Dagne K, Timko MP (2016) Assessment of genetic diversity in Ethiopian cowpea [Vigna unguiculata (L.) Walp.] germplasm using simple sequence repeat markers. Plant Mol Biol Report 34:978–992
Doumbia IZ, Akromah R, Asibuo JY (2014) Assessment of cowpea germplasms from Ghana and Mali using simple sequence repeat (SSR) markers. Int J Agric For 4:118–123
Egbadzor KF, Ofori K, Yeboah M, Aboagye LM, Opoku-Agyeman MO, Danquah EY, Offei SK (2014) Diversity in 113 cowpea [Vigna unguiculata (L) Walp] accessions assessed with 458 SNP markers. SpringerPlus 3:541
Ergun K, Yilmaz-Gokdogan E (2016) Using two retrotransposon based marker systems (IRAP and REMAP) for molecular characterization of olive. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 44:167
Gale M, Moore G, Devos K (2001) Rice—the pivotal genome in cereal comparative genetics. Rice Biotechnology: Improving Yield, Stress Tolerance and Grain Quality 236:46–58
Galyuon IK, Madhusudhana R, Borrell AK, Hash TC, Howarth CJ (2016) Genetic diversity of stay-green sorghums and their derivatives revealed by microsatellites. Afr J Biotechnol 15:1363–1374
Glaszmann J-C, Kilian B, Upadhyaya HD, Varshney RK (2010) Accessing genetic diversity for crop improvement. Curr Opin Plant Biol 13:167–173
Islam MM, Ali MS, Prodhan SH (2012) SSR marker-based molecular characterization and genetic diversity analysis of aromatic landreces of rice (Oryza sativa L.) Biosci Biotechnol 1:107–116
Kalendar R (2011) The use of retrotransposon-based molecular markers to analyze genetic diversity. Field Veg Crops Res 48:216–274
Kalendar R, Grob T, Regina M, Suoniemi A, Schulman A (1999) IRAP and REMAP: two new retrotransposon-based DNA fingerprinting techniques. Theor Appl Genet 98:704–711
Kalendar R, Flavell A, Ellis T, Sjakste T, Moisy C, Schulman AH (2011) Analysis of plant diversity with retrotransposon-based molecular markers. Heredity 106:520–530
Kouam EB et al (2012) Genetic structure and mating system of wild cowpea populations in West Africa. BMC Plant Biol 12:113
Kuruma R, Kiplagat O, Ateka E, Owuoche G (2008) Genetic diversity of Kenyan cowpea accessions based on morphological and microsatellite markers. East Afrr Agric For J 76:3–4
Li C-D, Fatokun CA, Ubi B, Singh BB, Scoles GJ (2001) Determining genetic similarities and relationships among cowpea breeding lines and cultivars by microsatellite markers. Crop Sci 41:189–197
Liu K, Muse SV (2005) PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129
Manifesto M, Schlatter A, Hopp H, Suarez E, Dubcovsky J (2001) Quantitative evaluation of genetic diversity in wheat germplasm using molecular markers. Crop Sci 41:682–690
Menz M, Klein R, Mullet J, Obert J, Unruh N, Klein P (2002) A high-density genetic map of Sorghum bicolor (L.) Moench based on 2926 AFLP®, RFLP and SSR markers. Plant Mol Biol 48:483–499
Molin D, Coelho C, Máximo D, Ferreira F, Gardingo J, Matiello R (2013) Genetic diversity in the germplasm of tropical maize landraces determined using molecular markers. Genet Mol Res 12:99–114
Nei M, Kumar S, Takahashi K (1998) The optimization principle in phylogenetic analysis tends to give incorrect topologies when the number of nucleotides or amino acids used is small. Proc Natl Acad Sci USA 95:12390–12397
Obembe OO (2008) Exciting times for cowpea genetic transformation research. Life Sci J 5:50–52
Oppong-Konadu E, Akromah H, Okai E (2005) Genetic diversity within Ghanaian cowpea germplasm based on SDS-page of seed proteins. Afr Crop Sci J 13:113–123
Otwe EP, Hesslop PH, Galyuon IKA (2011) Screening of Ghanaian cowpea [Vigna unguiculata (L) Walp.] for drought tolerance. In: Proceedings of the University of Ilorin, Nigeria, and University of Cape Coast, Ghana 2nd joint international conference on climate change and sustainable development. Ilorin. University of Ilorin, Nigeria, pp 390–504
Poehlman JM (2013) Breeding field crops. Springer Science & Business Media
Sajib AM et al (2012) SSR marker-based molecular characterization and genetic diversity analysis of aromatic landreces of rice (Oryza sativa L.) J Biosci Biotechnol 1:107–116
Salami HA et al. (2016) Genetic diversity of maize accessions (Zea mays l.) cultivated from benin using microsatellites markers. Am J Mol Biol 6:12
Sawadogo M, Ouedraogo JT, Gowda BS, Timko MP (2010) Genetic diversity of cowpea (Vigna unguiculata L. Walp) cultivars in Burkina Faso resistant to Striga gesnerioides. Afr J Biotechnol 9:8146–8153
Schulman AH (2007) Molecular markers to assess genetic diversity. Euphytica 158:313–321
Schulman AH, Flavell AJ, Paux E, Ellis TN (2012) The application of LTR retrotransposons as molecular markers in plants. Methods Mol Biol 859:115–153. doi:10.1007/978-1-61779-603-6_7
Sharawy WM, El-Fiky Z (2003) Characterization of cowpea (Vigna unguiculata L.) genotypes based on yield traits and RAPD-PCR analyses. Arab J Biotech 6:67–78
Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F (2000) Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J Exp Bot 51:1575–1584
Singh BP, Singh B, Mishra S, Kumar V, Singh NK (2016) Genetic diversity and population structure in Indian wild rice accessions. Aust J Crop Sci 10:144
Smith J et al (1997) An evaluation of the utility of SSR loci as molecular markers in maize (Zea mays L.): comparisons with data from RFLPs and pedigree. Theor Appl Genet 95:163–173
Sweeney M, McCOUCH S (2007) The complex history of the domestication of rice. Ann Bot 100:951–957
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A 101:11030–11035
Tan H, Tie M, Luo Q, Zhu Y, Lai J, Li H (2012) A review of molecular makers applied in Cowpea (Vigna unguiculata L. Walp.) Breeding. J Life Sci 6:1190
Timko MP, Singh B (2008) Cowpea, a multifunctional legume. Genomics of tropical crop plants. Springer, In, pp 227–258
Xiong H et al (2016) Genetic diversity and population structure of cowpea (Vigna unguiculata L. Walp). PLoS One 11:e0160941
Xu H, Jing T, Tomooka N, Kaga A, Isemura T, Vaughan D (2008) Genetic diversity of the azuki bean (Vigna angularis (Willd.) Ohwi & Ohashi) gene pool as assessed by SSR markers. Genome 51:728–738
Yuzbasioglu G, Yilmaz S, Marakli S, Gozukirmizi N (2016) Analysis of Hopi/Osr27 and Houba/Tos5/Osr13 retrotransposons in rice. Biotechnol Biotechnol Equip 30:213–218
Zargar SM, Sharma A, Sadhu A, Agrawal GK, Rakwal R (2014) Exploring genetic diversity in common bean from unexploited regions of Jammu & Kashmir-India. Mol Plant Breed 5:5–9
Zargar SM, Farhat S, Mahajan R, Bhakhri A, Sharma A (2016) Unraveling the efficiency of RAPD and SSR markers in diversity analysis and population structure estimation in common bean. Saudi J Biol Sci 23:139–149
Zhou R, Wu Z, Cao X, Jiang F (2015) Genetic diversity of cultivated and wild tomatoes revealed by morphological traits and SSR markers. Genet Mol Res 14:13868–13879
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by: Yuval Cohen
Rights and permissions
About this article
Cite this article
Otwe, E.P., Agyirifo, D.S., Galyuon, I.K. et al. Molecular Diversity in some Ghanaian Cowpea [Vigna unguiculata L. (Walp)] Accessions. Tropical Plant Biol. 10, 57–67 (2017). https://doi.org/10.1007/s12042-017-9184-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12042-017-9184-9







 = Drought Tolerant;
= Drought Tolerant;  = Drought Susceptible;
= Drought Susceptible;  = IITA and Leicester out-groups
= IITA and Leicester out-groups