Abstract
Haematopoiesis is a complex process in which the regulatory mechanisms of several implicated transcription factors remain uncertain. Drosophila melanogaster is an excellent model to resolve the unanswered questions about the blood cell development. This study describes the role of Beadex, a Drosophila homologue of LIM domain only 2 (LMO2), in haematopoiesis. Mutants of Beadex were analysed for blood cell abnormalities. Crystal cells, a subset of haemocytes, were significantly more in Beadex hypermorphic flies. Similarly, Beadex misexpression in prohemocytes altered the crystal cell numbers. Stage-specific misexpression analyses demonstrated that Beadex functions after the prohemocytes enter the crystal cell lineage. We also discovered that Pannier–U-shaped complex is a negative regulator of the crystal cell differentiation and is possibly negatively regulated by Beadex through its interaction with Pannier. We, therefore, suggest the mechanism of two novel regulators of crystal cell specification—Beadex and Pannier—during Drosophila haematopoiesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vertebrate haematopoiesis is a complex, well-studied process but the mechanisms of regulation by several important haematopoietic factors remain uncertain. LIM domain only protein-2 (LMO-2) is one such factor reported to regulate the embryonic erythropoiesis in mice (Warren et al. 1994; Yamada et al. 1998). LMO-2 partners with SCL/E2A and Ldb1 to inhibit erythroid differentiation (Visvader et al. 1997). However, during the erythropoiesis in Xenopus, LMO-2 with SCL and GATA-1 have been suggested to specify mesoderm to blood lineage (Mead et al. 2001). Deciphering the precise mechanism of regulation of vertebrate haematopoiesis by these factors has been a challenge.
Drosophila melanogaster shares the fundamental regulatory mechanisms and genetic control of haematopoiesis with vertebrates, making it an excellent model to resolve the unanswered questions in the blood development (Evans et al. 2003; Crozatier and Vincent 2011). Drosophila has three classes of blood cells: plasmatocytes, lamellocytes and crystal cells. The development of crystal cells is similar to the vertebrate erythroid development in the early phase, in terms of being the nonphagocytic cell (Palis and Yoder 2001) and the transcription factors involved, e.g. GATA factors, FOG factors, AML1/Runx1 etc. (Evans et al. 2003).
LMO-2 and Beadex mutants show similar phenotype conditions like ethanol and cocaine addiction (Heberlein et al. 2009; Lasek et al. 2011). Thus, understanding the role of Beadex in Drosophila haematopoiesis might give insights about the possible mode of LMO-2 function in vertebrate haematopoiesis. To study the role of LMO-2 in haematopoiesis, we analysed the mutants of Beadex (a Drosophila homologue of LMO-2), for blood cell abnormalities. Thereafter, we attempted to decipher the mechanism of regulation of haematopoiesis by Beadex.
Materials and methods
Drosophila strains and maintenance
Flies were reared on cornmeal–agar medium and maintained on a 12 h day/night cycle at 25°C. Canton-S was used as the wild-type strain and the other strains used in this study were: Bx1 (BS#15), BxJ (BS#3997), Bx7 (Kairamkonda and Nongthomba 2014), pnrD1 (BS#36551), He-Gal4 (BS#8700), Lz-Gal4 (BS#6314), UAS-Bx (a kind gift from S. M. Cohen, Denmark), UAS-BxRNAi (v2917, Vienna Drosophila RNAi Centre, Vienna), UAS-pnrWT (BS#7223), UAS-pnrRNAi (BS#34659) and UAS-pnrD4 (BS#36546). The BS numbers refer to stock numbers procured from the Bloomington Drosophila Stock Centre (BDSC, Indiana).
Total haemocyte number quantification
Two wandering third instar larvae of required genotype were bled into 5 µL of Schneider insect media. The extracted haemolymph was then smeared on a clean slide and incubated for 20 min at 25°C to allow the haemocytes to adhere on to the slide. Haemocytes were fixed on 2.5% paraformaldehyde for 15 min, washed with PBTx, and stained serially with 1:200 diluted phalloidin-FITC (P5282-FITC, 50 μg/mL stock; Sigma, India) for 15 min to label filamentous actin and propidium iodide (1 μg/mL; Sigma, India) to stain the nuclei. Slides were then washed gently with PBS to remove excess stain, mounted and observed under a fluorescent microscope (Olympus IX81). All the mountings were done using Vectashield mounting media (Vector Laboratories, USA). Ten randomly chosen fields from each slide were imaged and the numbers of cells were counted. Five slides were made for each genotype. Each experiment was repeated with biological replicates. Test and control samples were handled identically.
Crystal cell number quantification
Crystal cells are characterized by crystalline inclusions that contain the zymogen prophenoloxidase (proPO) and can be visualized by heating the larvae at 60°C for 15 min (Rizki et al. 1980). Wandering third instar larvae (n > 30) were treated with heat to visualize crystal cells. Melanized cells were counted in the three posterior abdominal segments (A6–A8). Crystal cells were imaged by an Olympus SZX12 stereomicroscope using an Olympus C-5060 camera. The data were plotted using GraphPad Prism 5. Statistical analyses were done using the Mann–Whitney test to estimate significance.
Results
Beadex mutants have abnormal numbers of haemocytes
Mutants of Bx were assessed for blood cell abnormalities. First, the total number of haemocytes was quantified. ‘Haemocyte count’ (a representation of the total number of haemocytes in the haemolymph) was defined as the average number of cells in 10 randomly chosen fields on a stained larval blood smear. Haemocyte counts of both Bx hypermorph mutant larvae (i.e. Bx1 and BxJ) were significantly lower than that of the wild type (figure 1, a&b). Similarly, overexpressing Bx, specifically in haemocytes also reduced the haemocyte count (figure 1b). The total haemocyte numbers were not altered when Beadex was knocked down using a haemocyte-specific Gal4 (He-Gal4). However, the knockdown of Beadex in the background of hypermorphic alleles rescued the haemocyte count to wild type levels (figure 1b), thus, showing a cell-autonomous effect of Beadex in regulating the total haemocyte number.
Beadex hypermorphs have fewer haemocytes. (a) Representative fluorescence images showing haemocytes in a blood smear in a randomly chosen field (40× magnifications). Cells were stained with phalloidin-FITC for visualizing cytoplasmic actin (green) while nuclei were stained with propidium odide. (b) Quantification of average haemocyte numbers from 10 larvae. Statistical analysis was done using one-way ANOVA followed by a post-test of Dunnett’s multiple comparisons. *Significant difference in haemocyte count of test vs wild type; #significant difference in haemocyte count of rescue vs mutant (background).
A decrease in the number of haemocytes could result from a defect in proliferation or specification. To determine the possible cause, the haemocyte subsets were quantified. Bx hypermorph larvae, Bx1 and BxJ, showed significantly higher numbers of crystal cells, while the null, Bx7, had fewer crystal cells (figure 2, a&b). While this was a dominant phenotype in the Bx hypermorphs (i.e. heterozygotes for the hypermorphic alleles showed increased crystal cell counts) the null, in this respect, was recessive (figure 2b). Further, knocking down the Beadex using He-Gal4, in the background of Beadex hypermorphic alleles, rescued the crystal cell counts partially in Bx1 and completely in BxJ (figure 2b). Thus, the effect of Beadex on the regulation of the crystal cell pool is specific to its expression in haemocytes.
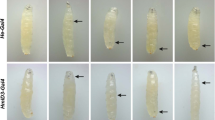
Crystal cells numbers vary in Beadex mutants. Wandering third instar larvae were heat-treated to visualize crystal cells (black dots). (a) Representative images of wild type and Beadex mutant larva after heat treatment showing the varied number of melanized crystal cells. (b) Quantitative representation of crystal cell counts in wild type, controls and Beadex mutant larvae (n ≥ 20). Knockdown of Beadex in haemocytes, using Hemese-Gal4 (He-Gal4), reduced crystal cell numbers in Beadex hypermorphs. The median is indicated by a black horizontal line. Statistical analyses to estimate significance were done using the Mann–Whitney test.
Misexpression of Beadex in the haemocytes affects crystal cell numbers
Since the crystal cell counts of genetic mutants were tested, it was important to check if the same effect could be seen with Beadex misexpression in the haemocytes alone. Indeed, haemocyte specific overexpression of Beadex (driven by He-Gal4) phenocopied the crystal cell counts of hypermorphs (i.e. increased crystal cell numbers) (figure 3). Likewise, knocking down of Beadex in haemocytes significantly decreased the crystal cell numbers, phenocopying the Beadex null, Bx7 (figures 2b&3). In addition, Beadex misexpression using another panhaemocyte driver hemolectin-Gal4 (hml-Gal4) yielded similar results (data not shown).
Beadex affects crystal cell numbers after their specification. Beadex was either knocked down or overexpressed in all haemocytes, using He-Gal4, or in specified crystal cells using Lz-Gal4. Similar to the mutants, overexpression or knockdown of Beadex leads to increased or decreased crystal cell counts, respectively. The median n ≥ 20 is indicated by a black horizontal line. Statistical analyses to estimate significance were done using the Mann–Whitney test.
Drosophila RUNX factor Lozenge (Lz) specifies crystal cells (Fossett et al. 2003; Waltzer et al. 2003; Ferjoux et al. 2007). Lz expression has been used as a marker of the crystal cell lineage (Gajewski et al. 2007). When Beadex was overexpressed using Lz-Gal4, crystal cell count increased dramatically (figure 3). Conversely, knockdown of Beadex using Lz-Gal4 reduced the crystal cell count (figure 3). Thus, Beadex effects its role during haematopoiesis after the commitment of blood cells to crystal cell lineage.
Misexpression of Pannier in haemocytes affects crystal cell numbers
GATA factor Serpent (Srp) has multiple roles to play in Drosophila haematopoiesis. One of them being its requirement, along with Notch and Lozenge, for crystal cell specification (Fossett et al. 2003). Pannier (Pnr), a known interacting partner of Beadex (Zenvirt et al. 2008), is another GATA factor in Drosophila (Ramain et al. 1993). However, the role of pnr in haematopoiesis has not been studied. Thus, before checking whether Beadex affects crystal cell specification through its interaction with Pnr, the effect of pnr on crystal cell development was tested using the same misexpression platform as above.
Surprisingly, contrary to previous reports on Srp (Fossett et al. 2003; Muratoglu et al. 2007), He-Gal4-driven overexpression of pnr decreased the crystal cell numbers (figure 4). Likewise, knockdown of pnr led to higher crystal cell numbers (figure 4). Similar results were obtained when pnr was misexpressed using Lz-Gal4 (figure 4). These results indicate that Pnr plays an inhibitory role in crystal cell development.
Pannier misexpression alters crystal cell numbers. He-Gal4 and Lz-Gal4 driven overexpression of pannier decreased crystal cell counts. He-Gal4 and Lz-Gal4 driven knockdown of pannier increased crystal cell counts. The median n ≥ 20 is indicated by a black horizontal line. Statistical analyses to estimate significance were done using the Mann–Whitney test.
Genetic interactions of Beadex and Pannier during crystal cell development
To further assess the plausible mechanism for the role of Beadex in crystal cell development, we checked the genetic interaction of Beadex and pnr during haematopoiesis. Compared to the counts in Bx1, we observed a significant increase in crystal cell number in Bx1 larvae which had pnr knocked down in their haemocytes (figure 5a). They were, in fact, as high as crystal cell counts of larvae with haemocyte-specific pnr knockdown (figure 5a). (P value of Mann–Whitney test: crystal cell count of [Bx1, He > pnrKD] vs [He > pnrKD] = 0.1677).
Beadex–pannier interactions. (a) Crystal cell counts of larvae with He-Gal4 driven pannier knockdown in Bx1 background, and of larvae with He-Gal4 driven overexpression of Beadex and pannier. The median n ≥ 20, is indicated by a black horizontal line. Statistical analyses to estimate significance were done using the Mann–Whitney test. (b) Approximate fold change in crystal cell numbers upon misexpression of pannier or Beadex and a hypothesis of possible epistatic interaction between Beadex and pannier during crystal cell development. Pannier misexpression masks the effect of Beadex on crystal cell numbers. GOF, gain of function; LOF, loss of function; Exp., expected fold change in crystal cell counts; Obs., observed fold change in crystal cell counts.
When Beadex and pnr were simultaneously overexpressed in haemocytes, the crystal cell counts of these larvae were low (figure 5a). In fact, the average number of crystal cells in these larvae was similar to those in larvae with pnr overexpression alone (figure 5a). (P value of Mann–Whitney test: crystal cell count of [He > pnrOE, BxOE] vs [He > pnrOE] = 0.0655).
Figure 5b explains the possible genetic interaction of Beadex and pnr in terms of fold change in crystal cell numbers. Individually, gain of function (GOF) of Beadex or loss of function (LOF) of pnr cause approximately four-fold increase in the crystal cell counts, whereas, LOF of Beadex or GOF of pnr lead to a reduction in crystal cell numbers. However, when GOF of both Beadex and pnr were brought together, the cell counts resembled that of pnr GOF alone. In addition, if GOF of Beadex and LOF of pnr were brought together, the crystal cell counts in such larvae were similar to LOF of pnr alone. Thus, pannier misexpression masks the effect of Beadex on crystal cell numbers. In other words, Pnr functions downstream of Beadex with respect to their action on crystal cell development.
Binding of Pannier to cofactor U-shaped (Ush) is necessary for its inhibitory action on crystal cell development
Pnr is a GATA factor that acts with a cofactor, the friend of GATA (FOG), Ush (Haenlin et al. 1997). Dominant mutant forms of pnr (pnrD1 and pnrD4) retain their DNA-binding capacity but are unable to bind to Ush (Haenlin et al. 1997). We next tested whether binding to Ush is important for the inhibitory function of Pnr during crystal cell development. Crystal cell counts of pnrD1 were high (figure 6), indicating the importance of Pnr binding to Ush during crystal cell development. Moreover, when another mutant form of pnr, also incapable of binding Ush (UAS-pnrD4), was overexpressed using haemocyte-specific Gal4 (He-Gal4), it led to an increase in crystal cell counts (figure 6). This was contrary to wild-type pnr overexpression but similar to pnr knockdown (see figure 4). Similar results were observed when the overexpression was driven in specified crystal cells using Lz-Gal4 (figure 6b).
Thus, the inhibitory role of Pnr in crystal cell development is dependent on its binding to Ush.
Discussion
This study reports a positive regulatory role of Beadex during crystal cell specification. GOF of Beadex leads to an increase in the crystal cell population (in hypermorphs and flies with Bx overexpression). The reverse is also true, i.e. loss of Beadex function reduces the crystal cell population both in mutants and knockdown flies. Altering Beadex expression after Lozenge (a crystal cell specification factor (Fossett et al. 2003; Evans et al. 2003; Williams 2007)) starts expressing, also results in crystal cell number defects, suggesting that the developmental point of action of Beadex is after the fate specification of crystal cell progenitors.
Not many players are known in crystal cell development. One of the important players is the GATA factor Serpent (Srp). Three of the six vertebrate GATA genes (GATA 1, GATA 2 and GATA 3) control haematopoiesis at various stages (Shimizu and Yamamoto 2005). Among the five Drosophila GATA genes, only Srp has been studied in detail with respect to haematopoiesis. Srp plays important roles at several stages of haematopoiesis, from haemocyte fate specification to terminal differentiation (Waltzer et al. 2010). Srp has a dual role in the crystal cell lineage. On the one hand, Srp is required for crystal cell differentiation in conjunction with Notch and the RUNX transcription factor Lozenge (Fossett et al. 2003; Waltzer et al. 2003; Ferjoux et al. 2007), but on the other hand, SrpNC (an alternatively spliced Srp isoform) represses crystal cell fate choice by associating with FOG factor Ush (Fossett et al. 2001; Gao et al. 2009).
Another Drosophila GATA factor, Pnr, is a cell-autonomous positive regulator of plasmatocyte differentiation (Minakhina et al. 2011). Minakhina et al. (2011) also found that knocking down pnr, in the cortical zone of lymph glands increased the crystal cell numbers in 30% of all cases. Similarly, we found an inhibitory role of Pnr in crystal cell development. Moreover, for this activity, Pnr needs to bind to its cofactor Ush. The inhibitory action of Pnr–Ush complex on crystal cell development is similar to the action of Srp–Ush. Thus, this study reports a novel negative regulatory complex of crystal cell differentiation. As of now, it is still not clear whether the inhibition occurs after Lz-Notch-Srp-driven specification or along with it. If the inhibition occurs after specification, this would be a unique example of in vivo despecification.
Finding differentiated crystal cells in pnr LOF clones, Minakhina et al. (2011) rejected the hypothesis that Pnr could affect crystal cell development. The small representation of crystal cells in the total haemocytes could have been responsible for the underestimation, as a minor increase in crystal cells would be difficult to notice in clones. Another possible explanation could be that Pnr may not play a major role in crystal cell development in the lymph gland.
Beadex and pnr regulate crystal cell differentiation in opposite ways; Beadex promotes, while pnr inhibits, crystal cell differentiation. Earlier reports have shown physical interaction between pnr and Beadex during sensory organ precursor specification (Asmar et al. 2008; Zenvirt et al. 2008). It is, thus, possible that during normal haematopoiesis, Beadex regulates the levels of Pnr–Ush inhibitory complex. In the Beadex GOF scenario, Beadex might compete with Ush for binding to Pnr, thereby disrupting the Pnr–Ush inhibitory complex. Similarly, under the Beadex LOF scenario, Pnr–Ush complex should be more stable due to reduced interactions of Beadex with Pnr (summarized in figure 7). This is in concurrence with what was observed during our genetic interaction studies, where pnr masked the effect of Beadex.
A proposed mechanism of action of Beadex and pannier in crystal cell development. Beadex is involved in crystal cell development after their specification. GATA factor Pannier (Pnr) inhibits crystal cell development. Binding to Ush is essential for Pnr mediated inhibition. Beadex regulates the inhibitory complex (Pnr–Ush).
While LMO has long been known to be associated with T-cell acute lymphoblastic leukaemia (Rabbitts 1998), it would be interesting to see if the oncogenic nature of mutant LMO is because of its inability to control the antidifferentiation action of GATA-FOG factors, similar to what has been reported in this study.
Contrary to the role of Beadex in crystal cell differentiation, the vertebrate homologue of Beadex, LMO2, along with its partner Ldb1, negatively regulates erythroid differentiation, thereby maintaining progenitor state (Visvader et al. 1997). Since the numbers of plasmatocytes were reduced in the Beadex mutants (indicated by lower total haemocytes but higher crystal cells), it would be interesting to study whether Beadex negatively regulates plasmatocyte specification or diverts prohemocytes, destined to be specified into plasmatocytes, to crystal cell lineage. Moreover, in mouse, xenopus and zebrafish, LMO2 was found to be essential at the early stages of blood development thereby causing early embryonic lethality (Warren et al. 1994; Yamada et al. 1998; Mead et al. 2001; Patterson et al. 2007). Since Drosophila does not depend on erythrocytes/blood for oxygen supply, we could study a function of LMO in the later stages of haematopoiesis. It would be interesting to see if LMO2 (or other LMOs) might have finetuning functions in later stages of haematopoiesis in the vertebrates as well. LMO2 is also shown to delay the expression of Runx in zebrafish (Patterson et al. 2007). Similarly, Beadex might have a temporal effect on Lz expression thereby delaying crystal cell development. This may explain a part of the phenotypes that we have seen in present study. However, since similar effect was seen when Beadex was misexpressed using Lz-Gal4, the major function of Beadex was after Runx/Lx expression.
References
Asmar J., Biryukova I. and Heitzler P. 2008 Drosophila dLMO-PA isoform acts as an early activator of achaete/scute proneural expression. Dev. Biol. 316, 487–497.
Crozatier M. and Vincent A. 2011 Drosophila: a model for studying genetic and molecular aspects of haematopoiesis and associated leukaemias. Dis. Model. Mech. 4, 439–445.
Evans C. J., Hartenstein V. and Banerjee U. 2003 Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell. 5, 673–690.
Ferjoux G., Auge B., Boyer K., Haenlin M. and Waltzer L. 2007 A GATA/RUNX cis-regulatory module couples Drosophila blood cell commitment and differentiation into crystal cells. Dev. Biol. 305, 726–734.
Fossett N., Hyman K., Gajewski K., Orkin S. H. and Schulz R. A. 2003 Combinatorial interactions of serpent, lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc. Natl. Acad. Sci. USA 100, 11451–11456.
Fossett N., Tevosian S. G., Gajewski K., Zhang Q., Orkin S. H. and Schulz R. A. 2001 The friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc. Natl. Acad. Sci. USA 98, 7342–7347.
Gajewski K. M., Sorrentino R. P., Lee J. H., Zhang Q., Russell M. and Schulz R. A. 2007 Identification of a crystal cell-specific enhancer of the black cells prophenoloxidase gene in Drosophila. Genesis 45, 200–207.
Gao H., Wu X. and Fossett N. 2009 Upregulation of the Drosophila friend of GATA gene U-shaped by JAK/STAT signaling maintains lymph gland prohemocyte potency. Mol. Cell. Biol. 29, 6086–6096.
Haenlin M., Cubadda Y., Blondeau F., Heitzler P., Lutz Y., Simpson P. and Ramain P. 1997 Transcriptional activity of pannier is regulated negatively by heterodimerization of the GATA DNA-binding domain with a cofactor encoded by the u-shaped gene of Drosophila. Genes Dev. 11, 3096–3108.
Heberlein U., Tsai L. T., Kapfhamer D. and Lasek A. W. 2009 Drosophila, a genetic model system to study cocaine-related behaviors: a review with focus on LIM-only proteins. Neuropharmacology 56 (suppl 1), 97–106.
Kairamkonda S. and Nongthomba U. 2014 Beadex function in the motor neurons is essential for female reproduction in Drosophila melanogaster. PLoS One 9, e113003.
Lasek A. W., Giorgetti F., Berger K. H., Tayor S. and Heberlein U. 2011 Lmo genes regulate behavioral responses to ethanol in Drosophila melanogaster and the mouse. Alcohol. Clin. Exp. Res. 35, 1600–1606.
Mead P. E., Deconinck A. E., Huber T. L., Orkin S. H. and Zon L. I. 2001 Primitive erythropoiesis in the Xenopus embryo: the synergistic role of LMO-2, SCL and GATA-binding proteins. Development 128, 2301–2308.
Minakhina S., Tan, W. and Steward R. 2011 JAK/STAT and the GATA factor Pannier control hemocyte maturation and differentiation in Drosophila. Dev. Biol. 352, 308–316.
Muratoglu S., Hough B., Mon S. T. and Fossett N. 2007 The GATA factor Serpent cross-regulates lozenge and u-shaped expression during Drosophila blood cell development. Dev. Biol. 311, 636–649.
Palis J. and Yoder M. C. 2001. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 29, 927–936.
Patterson L. J., Gering M., Eckfeldt C. E., Green A. R., Verfaillie C. M., Ekker S. C. et al. 2007 The transcription factors Scl and Lmo2 act together during development of the hemangioblast in zebrafish. Blood 109, 2389–2398.
Rabbitts T. H. 1998 LMO T-cell translocation oncogenes typify genes activated by chromosomal translocations that alter transcription and developmental processes. Genes Dev. 12, 2651–2657.
Ramain P., Heitzler P., Haenlin M. and Simpson P. 1993 Pannier, a negative regulator of achaete and scute in Drosophila, encodes a zinc finger protein with homology to the vertebrate transcription factor GATA-1. Development 119, 1277–1291.
Rizki T. M., Rizki R. M. and Grell E. H. 1980 A mutant affecting the crystal cells in Drosophila melanogaster. Wilehm Roux Arch. 188, 91–99.
Shimizu R. and Yamamoto M. 2005 Gene expression regulation and domain function of hematopoietic GATA factors. Semin. Cell. Dev. Biol. 16, 129–136.
Visvader J. E., Mao X., Fujiwara Y., Hahm K. and Orkin S. H. 1997 The LIM-domain binding protein Ldb1 and its partner LMO2 act as negative regulators of erythroid differentiation. Proc. Natl. Acad. Sci. USA 94, 13707–13712.
Waltzer L., Ferjoux G., Bataille L. and Haenlin M. 2003 Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. EMBO J. 22, 6516–6525.
Waltzer L., Gobert V., Osman D. and Haenlin M. 2010 Transcription factor interplay during Drosophila haematopoiesis. Int. J. Dev. Biol. 54, 1107–1115.
Warren A. J., Colledge W. H., Carlton M. B., Evans M. J., Smith A. J. and Rabbitts T. H. 1994 The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell 78, 45–57.
Williams M. J. 2007 Drosophila hemopoiesis and cellular immunity. J. Immunol. 178, 4711–4716.
Yamada Y., Warren A. J., Dobson C., Forster A., Pannell R. and Rabbitts T. H. 1998 The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc. Natl. Acad. Sci. USA 95, 3890–3895.
Zenvirt S., Nevo-Caspi Y., Rencus-Lazar S. and Segal D. 2008 Drosophila LIM-only is a positive regulator of transcription during thoracic bristle development. Genetics 179, 1989–1999.
Acknowledgments
We thank Amartya Mukherjee for help with editing. We acknowledge the Indian Institute of Science (IISc), Department of Science and Technology (DST) (DST FIST, 2008 – 2013 ref. no. SR/FST/LSII-018/2007), the University Grant Commission (UGC-SAP to MRDG: ref. no. F.3-47/2009 (SAP-II) and the Department of Biotechnology (DBT), Govt. of India, (DBT-IISC Partnership Programme for Advanced Research in Biological Sciences and Bioengineering sanction order no: DBT/BF/PRIns/2011-12/IISc/28.9.2012) for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: H. A. Ranganath
Rights and permissions
About this article
Cite this article
CHATTERJEE, A., AAVULA, K. & NONGTHOMBA, U. Beadex, a homologue of the vertebrate LIM domain only protein, is a novel regulator of crystal cell development in Drosophila melanogaster. J Genet 98, 107 (2019). https://doi.org/10.1007/s12041-019-1154-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12041-019-1154-6