Abstract
A striking infertile phenotype has been discovered in the DDK strain of mouse. The DDK females are usually infertile when crossed with males of other inbred strains, whereas DDK males exhibit normal fertility in reciprocal crosses. This phenomenon is caused by mutation in the ovum (Om) locus on chromosome 11 and known as the DDK syndrome. Previously, some research groups reported that the embryonic mortality deviated from the semilethal rate in backcrosses between heterozygous (Om/ + ) females and males of other strains. This embryonic mortality exhibited an aggravated trend with increasing background genes of other strains. These results indicated that some modifier genes of Om were present in other strains. In the present study, a population of N2 (Om/ + ) females from the backcrosses between C57BL/6J (B6) and F1 (B6 ♀ × DDK ♂) was used to map potential modifier genes of Om. Quantitative trait locus showed that a major locus, namely Amom1 (aggravate modifier gene of Om 1), was located at the middle part of chromosome 9 in mice. The Amom1 could increase the expressivity of Om gene, thereby aggravating embryonic lethality when heterozygous (Om/ +) females mated with males of B6 strain. Further, the 1.5 LOD-drop analysis indicated that the confidence interval was between 37.54 and 44.46 cM, ∼6.92 cM. Amom1 is the first modifier gene of Om in the B6 background.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DDK inbred strain was cultivated by Japanese scholars from Mus musculus domesticus subspecies of Germany (Goto et al. 1979; Festing 1994; Zhao et al. 2002). It has a polar-infertile phenotype i.e., the DDK females are completely or nearly infertile when mated with males of other strains, whereas the crosses between DDK males and nonDDK females show normal fertility (Tomita 1960; Wakasugi et al. 1967). To explain this phenomenon, Wakasugi (1974) hypothesized that the polar infertility is caused by an incompatibility system between the DDK cytoplasmic factor and the nonDDK sperm factor, both of which are controlled by an allele of the gene named Ovum mutant (Om). In most strains, wild type of Om produces a cytoplasmic factor ‘O’ in females and a sperm factor ‘S’ in males during gametogenesis, whereas in the DDK strain, it produces a cytoplasmic factor ‘o’ in ovary and a sperm factor ‘s’ in sperm. When o and S are combined together (o–S), the embryos develop abnormal lethality, while those having other three combinations, O–S, O–s and o–s, develop normally. This hypothesis has been confirmed by transfer experiments of pronuclei and cytoplasm (Mann 1986; Babinet et al. 1990), and the Om locus is located on chromosome 11 at 47 cM (Baldacci et al. 1992, 1996; Sapienza et al. 1992; Cohen-Tannoudji et al. 2000). Further, the DDK cytoplasm factor has been identified as RNA in oocytes (Renard et al. 1994).
According to Wakasugi’s incompatibility theory, the embryonic mortality produced from backcrosses, F1 (Om/ + ) ♀ × Alien (+ / + ) ♂ and DDK (Om/Om) ♀ × F1 (Om/ + ) ♂, should be half the rate of those from the normal crosses, B6 (+ / + ) ♀ × DDK (Om/Om) ♂. However, some research groups reported that the actual reproductive rate deviated significantly from the theoretical rate of 50% in crosses between heterozygous (Om/ +) females and males (+ / +) of the B6 or BALB/c strain. Pardo-Manuel de Villena et al. (1999) presented evidence supporting existence of certain modifier genes, which could decrease the reproductive rate when N2 heterozygous (Om/ + ) females mated with B6 males. Le Bras et al. (2000) proposed that some modifier genes of Om exist in the genetic background of the BALB/c strain, which could increase the mortality of offspring when the N2 heterozygous (Om/ + ) females mated with males of the BALB/c strain. Zhao et al. (2000) also claimed that the embryonic mortality of heterozygous (Om/ +) females showed a stepwise increase in successive backcrosses with males of the B6 strain indicating presence of some modifier genes of Om existing in the genetic background of the B6 strain. The purpose of this present study was to locate modifier genes of Om through a linkage analysis between the genotype and the phenotype for N2 heterozygous (Om/ +) females backcrossed with males of the B6 strain.
Materials and methods
Animals and general care
The laboratory strains used in this study were DDK, provided by BioReasource Center, RIKEN (Tsukuba, Ibaraki, Japan), and C57BL/6J (B6), purchased from Henan Laboratory Animal Center (Zhengzhou, China). Production colonies were developed with the introduced individuals and their descendants were used for experiments. Animals were cared under controlled conditions as described previously (Zhao et al. 2000). The female mice used in this study were 2–4 months old and the males were 2–6 months old. All animal procedures were performed according to the guidelines of Animal Experimentation of HENAN Agriculture University. The following gene symbols are used in this report: DDK allele, Om; all other alleles, + .
Mating procedure and the screening of N 2 heterozygous (Om/ + ) females
F1 (B6 ♀ × DDK ♂) were backcrossed to B6 mice and the female offspring (N2) were screened using three microsatellite markers linked to the Om locus (D11Mit36, D11Mit66 and D11Mit247), to identify heterozygous females. Females in the crosses of ((Om/ +) N2♀ × B6 ♂) were checked daily for vaginal plugs and dissected at 12–15 days of pregnancy to record the number of implantations (IM) and live foetus (LF) phenotypes. As shown in figure 1, the normal embryos were recorded as the number of LF and the number of IM were counted including live and dead ones.
PCR and genotype determination
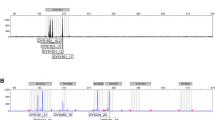
Two hundred (Om/ + ) N2 individuals were used for a whole-genome scan by polymorphic microsatellite markers on the 19 autosomes. Subsequently, 289 samples including 200 N2 (Om/ +) individuals of whole-genome scan were used for fine mapping on chromosome 9. Microsatellite markers were selected from the Mouse Genome Informatics database (http://www.informatics.jax.org/) and the primers were synthesized by Takara (Tokyo, Japan). The polymorphic markers were obtained through PCR with genomic DNA of B6, DDK and F1 mice.
Genome DNA was extracted from the tails of 21-day-old mice using the TailGen DNA kit (CoWin Biotech, China), and genotypes were determined by PCR according to the procedure of Routman and Cheverud (1994) in 96-well microtitration plates on a Mastercycler gradient B5331 instrument (Eppendorf, Hamburg, Germany). PCR products were size fractionated by electrophoresis on 3.5% agarose gels (120 × 60 × 4 mm) in 0.5 × TBE buffer at 100 V for 1.5 h. Gels were stained with ethidium bromide and photographed under UV light.
Quantitative trait loci (QTL) analysis
The LF and IM of N2 individuals were recorded for gene mapping and the QTL analysis was performed with Haley–Knott regression to analyse the QTL in R/qtl software and to calculate the LOD threshold by 1000 permutation method (Haley and Knott 1992). To locate modifier genes, two analyses were used in this study. First, a whole-genome scan was performed as an initial analysis to ascertain the chromosome on which the locus was located. Second, according to the results of the whole-genome scan, the fine mapping analysis was conducted on chromosome 9. We used 1.5 LOD-drop support intervals to estimate the confidence interval of significant locus after locating loci’s significant association with the phenotype the LOD-drop correspond to about 95% confidence intervals (Dupuis and Siegmund 1999).
Results
Screening N 2 heterozygous (Om/ + ) females
As crosses between DDK females and B6 males are sterile, mapping populations were obtained from F1 (B6 ♀×DDK♂) × B6 backcrosses. We collected 1245 N2 offspring that included 608 N2 females. Using three microsatellite markers (D11Mit36, D11Mit66 and D11Mit247) 298 N2 heterozygous (Om/ +) females were selected that are closely linked to the Om locus. Three N2 heterozygous females died before mating, and six N2 heterozygous females died in the mating experiment; only 289 N2 heterozygous females were selected for gene mapping.
Phenotype of N 2 mice
The N2 heterozygous (Om/ + ) females were mated with B6 males and examined for the number of IM and LF at 12–15 days of pregnancy. The reproductive performance in the backcrosses of (F1♀ (B6 ♀×DDK♂) × B6 ♂) (n = 31) and (N2♀ × B6 ♂) (n = 289) were recorded to compare phenotype distribution of heterozygous females in different backcross generations (figure 2). Both sets of phenotypic data exhibited a normal distribution by the Kolmogorov–Smirnov test (P>0.05) and the reproductive rate of N2 heterozygous females exhibited a decreasing trend. These results showed that there may be some modifier genes of Om present in the genetic background of the B6 strain.
Examining polymorphism markers and genotype of N 2 samples
Markers 55, that are polymorphic for DDK and B6 strains were selected from 338 candidate microsatellite markers that are distributed on 19 autosomes. Sex chromosomes were not included as there was no relationship between gender and genetic modification of Om. Based on the results of the whole-genome scan, 16 markers on chromosome 9 were chosen for fine mapping in the N2 mice.
QTL analysis
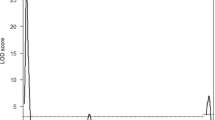
The association analysis was performed between the fertility data and the genotype data from the N2 heterozygous (Om/ +) females (see figure 3a). Using the R/qtl software, the significance threshold of the whole-genome scan was found to be 2.68 for LF. The LOD scores (3.16) indicated that a modifier locus of LF was located on chromosome 9, close to D9Mit273 microsatellite marker (49 cM). The maximum LOD score of IM on chromosome 9 was 1.73, not exceeding the 5% significance threshold level of 2.5. This means that there was no locus of IM on chromosome 9. Therefore, only one locus of LF on chromosome 9 was established for the fine mapping.
(a) Result of QTL analysis. Whole-genome scan analysis of the N2 heterozygous females yielded a significant modifier locus on chromosome 9. Sex chromosomes were not included in the analysis as there was no relationship between gender and genetic modification of Om. The black lines indicate the genomewide scan result of LF; the red lines indicate the genomewide scan result of LM. Genomewide significance thresholds are indicated at α = 0.05 (dashed line). (b) LOD scores for the QTLs on chromosome 9. The horizontal dashed line indicate the 5% significance threshold level (α = 0.05).
For the fine mapping, an additional 12 polymorphic markers were selected for detecting 289 N2 samples. The maximum LOD score (3.37) was observed near the D9Mit73 microsatellite marker (39.85 cM) (figure 3b). We still used the genomewide significance threshold levels, the LOD score was 2.68 (P = 0.05) for LF. A confidence interval for this major locus of LF was ∼6.92 cM, including the region from 37.54 to 44.46 cM. According to the QTL effect analysis, all N2 samples are partitioned into two phenotypic classes with their genotypes at D9Mit73 (figure 4). The LF of individuals with the homozygous genotype at the D9Mit73 locus (2.32 ± 0.31) was significantly lower than the heterozygous genotype (386 ± 0.24) (P<0.05). The QTL analysis (figures 3 and 4) indicated that there exists a modifier gene of Om in the genetic background of the B6 strain , named aggravate modifier gene of Ovum mutant 1 (Amom1).
Discussion
In the recent years, a number of researches have focussed on the DDK syndrome and the Om gene. However, many basic problems have remained unsolved till today, such as molecular mechanism of the DDK syndrome, the genetic sequence of Om, etc. Especially, modifier genes of Om have been found in the genetic background of some nonDDK strains, but the location and the number of modifier genes are still unknown (Pardo-Manuel de Villena et al. 1999; Zhao et al. 2000). The data presented in this study shows that a major locus located on the mid part of chromosome 9, named Amom1, is the first modifier gene of Om in the B6 background, which could aggravate embryo mortality in heterozygous (Om/ + ) females mated with males of the B6 strain.
The modifier genes could lead to a modified phenotype through altering the expression of their target genes (Romeo and McKusick 1994). According to the theory of Nadeau (2001), modifier genes are able to affect the penetrance, expressivity or pleiotropy of genes, and this influence on expressivity results in a distribution of the modified phenotype which becomes more extreme when the heterozygosis or homozygosis have the additional modifier genes. As seen in figure 2, the number of live foetus in the N2 heterozygous (Om/ +) females decreased. This decrease resulted in a shift in the distribution of the number of LF to the left with the B6 background genes increasing (Zhao et al. 2000). These results imply that there are some potential modifier genes of Om that exert an influence on the expressivity of Om.
In the present study, only one major locus was located in the live foetus, and the maximum LOD was 3.16. According to the theoretical postulation, there exist two types of genotypes in N2 females for the modifier gene (B6/B6) or (DDK/DDK). If only one modifier gene is present in the genetic background of the B6 strain, the number of LF in the population of N2 heterozygous (Om/ + ) females would present two peaks when backcrossed with B6 males. However, figure 2 shows the distribution of live foetus in the N2 heterozygous (Om/ + ) females exhibits a normal distribution, and there are no two peaks. This result suggested that there may be two or more modifier genes of Om present in the genetic background of the B6 strain. The confidence interval was ∼6.92 cM, from 37.54 to 44.46 cM, for the modifier locus on chromosome 9, and this interval was too broad for fine mapping. This broad result can be explained for the following two reasons: first, the sample sizes were insufficient; and second, the density of microsatellite markers was low. In the present study, only 55 polymorphic markers were chosen for the whole-genome scan and the average numbers of markers are less than three on each chromosome. The screening rate of polymorphic markers is lower than 16%, and this low efficiency was mainly caused by both B6 and DDK mice that belong to the same subspecies (M. m. domesticus). It is difficult to screen polymorphic markers between these two inbred strains due to their close genetic relationship (Goto et al. 1982; Zhao et al. 2002). Therefore, relying on the released markers would not be enough for meeting the requirements of further mapping of the modifier genes of Om. Mapping of some modifier genes may be missed because the genome covering rate of the 55 markers genotyped here was very low, i.e. only about 3.70%. To find more new polymorphic markers between the DDK and B6 mice is one of the important works and will produce a more useful effect for finding other potential modifier genes in the genetic background of the B6 strain.
For locating the modifier genes of Om, a number of corpora lutea, IM and LF were recorded in the present study. The number of corpora lutea of (F1♀ (B6 ♀×DDK♂) × B6 ♂) (n = 23) was 10.7 and (N2♀ × B6 ♂) (n = 89) was 10.5 Therefore, there was no significant difference between F1 and N2 backcrosses which is same as Zhao et al. (2000). Only the numbers of implantation and LF were recorded and were used for the whole-genome scan. The mapping results show that one major locus was mapped on chromosome 9 for the number of LF (the maximum LOD = 3.16), whereas no major loci was mapped for the number of IM (the maximum LOD = 1.73).
It was confirmed that the modifier genes of Om in the genetic background of the B6 strain could increase embryonic death at the same stage as the DDK syndrome (Zhao et al. 2000). The reduction in fertility of the DDK syndrome is due to the death of embryos which occurred during the 3rd–5th day of pregnancy, before or soon after implantation (Wakasugi et al. 1967, 1973). In the present study, the embryos death was determined at the 12–15th day of pregnancy, and the number of IM were always greater than or equal to those of LF in the N2 (Om/ + ) samples. Thus, we speculate that the modifier genes of Om not only have an impact on the preimplantation stage but also influence the embryo survival in the postimplantation, which is similar to the DDK syndrome.
The modifier gene of Om located in this study only aggravates the embryonic lethality in the N2 heterozygous (Om/ + ) females mated with males of the B6 strain. We noticed that Ideraabdullah et al. (2007) reported a modifier gene, named rescue modifier of the DDK syndrome 1 (Rmod1), located on chromosome 13 in the PERA and PERC mice which are wild-derived strains of M. m. domesticus and originated from Peru (Ideraabdullah et al. 2004). Rmod1 also leads to the embryonic mortality deviated from the semilethal rate in backcrosses between heterozygous (Om/ + ) females and nonDDK males such as the PERA and PERC. However, this deviation exhibited a completely opposite trend; in other words, Rmod1 is a rescue gene. Actually, Wakasugi (1974) already showed that there are some modifier genes which could alleviate the phenotype of the DDK syndrome in crosses between DDK and NC strain. NC inbred strain was cultivated from Japanese fancy mice (Nishiki-Nezumi), which belonged to M. m. molossinus subspecies (Kondo and Esaki 1961). Subsequently, Zhao et al. (2002) reported that DDK females are fully fertile when crossed to males of MOM (M. m. molossinus) and CASP (M. m. castaneus) strains. Song et al. (2011) also presented that DDK females are fully fertile when crossed with PWK, which belongs to M. m. musculus subspecies and is same as the PERA and PERC mice (Ideraabdullah et al. 2007). However, that work did not discuss whether there existed the possibility of modifier genes. Based on the above analysis, it is possible that the aggravate modifier gene of Om located in the present study is not related to those rescue modifier genes of Om.
References
Babinet C., Richoux V., Guénet J. L. and Renard J. P. 1990 The DDK inbred strain as a model for the study of interactions between parental genomes and egg cytoplasm in mouse preimplantation development. Dev. Suppl. 81–87.
Baldacci P. A., Richoux V., Renard J. P., Guénet J. L. and Babinet C. 1992 The locus Om, responsible for the DDK syndrome, maps close to Sigje on mouse chromosome 11. Mamm Genome 2, 100–105.
Baldacci P. A., Cohen-Tannoudji M., Kress C., Pournin S. and Babinet C. 1996 A high-resolution map around the locus Om on mouse chromosome 11. Mamm Genome 7, 114–116.
Cohen-Tannoudji M., Vandormael-Pournin S., Le Bras S., Coumailleau, F., Babinet C. and Baldacci P. 2000 A 2-Mb YAC/BAC-based physical map of the Ovum mutant (Om) locus region on mouse chromosome 11. Genomics 68, 273–282.
Dupuis J. and Siegmund D. 1999 Statistical methods for mapping quantitative trait loci from a dense set of markers. Genetics 151, 373–386.
Festing M. F. W. 1994 Inbred strains of mice. Mouse Genome 92, 373–495.
Goto N., Noguchi K. and Imamura K. 1979 Mouse strain identification by means of discriminate analysis using mandible measurements. Natl. Inst. Anim. Health Q (Tokyo) 19, 121–131.
Goto N., Miura K., Imamura K. and Komeda K. 1982 Genetic relationships among 5 inbred strains established from common ancestor, dd mouse, as assessed by mandible analysis. Natl. Inst. Anim. Health Q (Tokyo) 22, 70–75.
Haley C. S. and Knott S A. 1992 A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity 19, 315–324.
Ideraabdullah F. Y., de la Casa-Esperón E., Bell T. A., Detwiler D. A., Magnuson T., Sapienza C. et al. 2004 Genetic and haplotype diversity among wild-derived mouse inbred strains. Genome Res. 14, 1880–1887.
Ideraabdullah F. Y., Kim K., Pomp D., Moran J. L., Beier D. and Villena F. 2007 Rescue of the mouse DDK syndrome by parent-of-origin-dependent modifiers. Biol. Reprod. 76, 286–293.
Kondo K. and Esaki K. 1961 Breeding of tester strains for coat colour genes. Bull. Exp. Anim. 11, 194–196.
Le Bras S., Cohen-Tannoudji M., Kress C., Vandormael-Pournin S., Babinet C. and Baldacci P. 2000 BALB/c alleles at modifier loci increase the severity of the maternal effect of the “DDK syndrome”. Genetics 154, 803–811.
Mann J. R. 1986 DDK egg–foreign sperm incompatibility in mice is not between the pronuclei. J. Reprod. Fertil. 76, 779–781.
Mouse Genome Informatics database, http://www.informatics.jax.org/ (11 September 2012).
Nadeau J. H. 2001 Modifier genes in mice and humans. Nat. Rev. Genet. 2, 165–174.
Pardo-Manuel de Villena F., De la Casa-Esperon E., Verner A., Morgan K. and Sapienza C. 1999 The maternal DDK syndrome phenotype is determined by modifier genes that are not linked to Om. Mamm Genome 10, 492–497.
Renard J. -P., Baldacci P., Richoux-Duranthon V., Pournin S. and Babinet C. 1994 A maternal factor affecting mouse blastocyst formation. Development 120, 797–802.
Romeo G. and McKusick V. A. 1994 Phenotypic diversity, allelic series and modifier genes. Nat. Genet. 7, 451–453.
Routman E. and Cheverud J. 1994 A rapid method of scoring sample sequence repeat polymorphisms with agarose gel electrophoresis. Mamm Genome 5, 187–188.
Sapienza C., Paquette J., Pannunzio P., Albrechtson S. and Morgan K. 1992 The polar-lethal Ovum mutant gene maps to the distal portion of mouse chromosome 11. Genetics 132, 241–246.
Song G. D., Wang T. T., Guo J., Lei J., Li C. L., Zheng Z. Y. et al. 2011 Identification of compatibility between ooplasmic factor and sperm gene in the intersubspecific crosses involving DDK and PWK mice strains. J. Genet. Genomics 38, 525–531.
Tomita T. 1960 One-side cross sterility between inbred strains of mice. Japanese J. Genet. 35, 291.
Wakasugi N. 1973 Studies on fertility of DDK mice: reciprocal crosses between DDK and C57BL/6J strains and experimental transplantation of the ovary. J. Reprod. Fertil. 33, 283–291.
Wakasugi N. 1974 A genetically determined incompatibility system between spermatozoa and eggs leading to embryonic death in mice. J. Reprod. Fertil. 41, 85–96.
Wakasugi N., Tomita T. and Kondo K. 1967 Differences of fertility in reciprocal crosses between inbred strains of mice: DDK, KK and NC. J. Reprod. Fertil. 13, 41–50.
Zhao W. D., Chung H. J. and Wakasugi N. 2000 Modification of survival rate of mouse embryos developing in heterozygous females for ovum mutant gene. Biol. Reprod. 62, 857–863.
Zhao W. D., Ishikawa A., Yamagata T., Bolor H. and Wakasugi N. 2002 Female mice of DDK strain are fully fertile in the intersubspecific crosses with Mus musculus molossinus and Mus musculus castaneus. Mamm. Genome 13, 345–351.
Acknowledgement
This work was supported by National Natural Science Foundation of China (no. 30570267).
Author information
Authors and Affiliations
Corresponding author
Additional information
[Tan J., Song G. D., Song J. S., Ren S. H., Li C. L., Zheng Z. Y. and Zhao W. D. 2016 Locating a modifier gene of Ovum mutant through crosses between DDK and C57BL/6J inbred strains in mice. J. Genet. 95, xx–xx]
Jing Tan and Gendi Song contributed equally to this work.
Rights and permissions
About this article
Cite this article
TAN, J., SONG, G.D., SONG, J.S. et al. Locating a modifier gene of Ovum mutant through crosses between DDK and C57BL/6J inbred strains in mice. J Genet 95, 297–302 (2016). https://doi.org/10.1007/s12041-016-0633-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-016-0633-2