Abstract
Electro-crystallization, the freezing of water droplet induced by an electric field has been investigated by many investigators previously. But disagreements regarding the cause of freezing still persist in the literature. A cloud chamber of the internal dimension of 1 ft \(\times \) 1 ft has been designed to study electro-crystallization of ‘mm’ size pure water drops. More than 150 experiments have been performed in the chamber in the absence and presence of an electric field. Preliminary results suggest that in normal conditions, maximum drops freeze in the temperature ranging from −10° to –15°C, consistent with the previous laboratory studies. When the drops are subjected to an electric field of magnitude 2–5 kV cm−1, the drops are observed to freeze in a much warmer temperature ranging from –6° to –10°C indicating an electric field induced crystallization. No movement of the drops is observed during the freezing, which suggests that the freezing may be initiated by absorption of the latent heat of fusion by the Nylon wire where the drops are kept suspended. The implication of the electrically induced freezing from the perspective of cloud physics also has been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is well known that ice phase hydrometeors play a major role in cloud electrification through non-inductive charging mechanism (Takahashi 1978; Stolzenburg et al. 1998; Bruning et al. 2010) and also modify the surface precipitation through cold rain microphysics. But few studies are available concerning the growth behaviour of ice-phased hydrometeors in the mixed-phased region of the cloud once the clouds become strongly electrified. How the in-cloud electric field is going to affect the freezing of supercooled droplets and the subsequent growth or decay of the ice particles? Also, to understand the Earth’s climate system, the underlying cloud microphysical processes (both warm and cold) must be understood. Also, complex growth processes and inter-changeable habitat of the frozen hydrometeors make it difficult to study their growth behaviour in natural clouds. However, laboratory investigations provide a unique opportunity to understand the underlying micro-scale processes of a macro system like clouds. It is well known that the formation of ice crystals in the Earth’s atmosphere is often catalyzed by different factors, the primary one is the types and chemical characteristics of aerosol particles. Numerous laboratory investigation has been carried out to study the effect of electrical influences in water droplets break up, evaporation, oscillation and coalescence (Rayleigh 1879; Taylor 1964; Ausman and Brook 1967; Richards and Dawson 1971; Kamra and Ahire 1989; Bhalwankar et al. 2004; Bhalwankar and Kamra 2007). For example, Taylor (1964) observed that electric field could induce disintegration of water drops. Kamra and Ahire (1989) observed that an external electric field could change the shape of a water drop falling at its terminal velocity. Bhalwankar et al. (2004) observed that the rate of evaporation of charged drops is less than uncharged drops of the same size. It has been also observed that the presence of charge on the surface of water drops reduces the equilibrium saturation vapour pressure stabilizing the drop at subsaturated environments (Lapshin et al. 2002; Nielsen et al. 2011).
The uncertainty in the accurate prediction of ice phase hydrometeors (ice, graupel, and snow) produces major uncertainty in the simulation fields (Morrison et al. 2009). But as mentioned, few laboratory studies investigating the effect of the electrical force in ice crystals are available in the literature. Pruppacher and Klett (1996) reported that inside a strongly electrified cloud, the number concentration of ice crystals is found to be several orders of magnitude greater than measured ice nuclei and suggested that some secondary crystal generation mechanism must operate inside the cloud. In a cloud chamber investigation, Pruppacher (1963) observed enhanced ice-nucleation in the presence of electrostatically charged surfaces and external electric field. He proposed that electrical relief of the surface of a solid substrate (and not the crystallochemical relief) is inducing the heterogeneous ice nucleation in the presence of electric force. Abbas and Latham (1969) reported electro-freezing of a water drop in a temperature range of −5° to −20°C, if the drops are disrupted by an external electric field. They suggested that electro-freezing is associated with the movements of triple-phase boundary and the cause of freezing was attributed to the emanation of a filament structure from the drops which contain molecular aggregates that act as freezing nuclei. Schaefer (1968) and Salt (1961) suggested that ice nucleation of super-cooled water drops may take place through impurities produced by electric discharge. Mandal and Pradeep Kumar (2002) observed ice nucleation in a cold room experiment through corona discharge and suggested that the nucleation was possibly caused by the large number of ions produced during the discharge. They did not detect any nucleation by application of the only high electric field. In a cloud chamber experiment, Anderson et al. (1980) observed that ice particles that preferentially form on ions have a greater chance of growing to a larger size. A wind tunnel experiment of electro-freezing by Dawson and Cardell (1973) suggest no substantial influence of vertical electric field in the enhancement of freezing of super-cooled water drops in the temperature range −8° to −15°C.
Although there is a few experimental evidence of electro-crystallizaition with an application of an external electric field available in the literature, it may be noted that in this set of experiments, the drops are subjected to electrical or mechanical disturbances. The possibility of electro-crystallization without disturbing the drops has not been investigated till now. A proper understanding of this aspect is important as the freezing of super-cooled droplets at a higher temperature will generate a significant amount of latent heat which may in turn, impact the dynamics of the thunderclouds. A proper understanding of the freezing characteristics along with the crystal habitat will help to reduce the uncertainty associated with ice hydrometeors in weather/climate models. To investigate the effect of an external electric field in electro-crystallization process along with the shape and size of crystals, a cloud chamber experiment has been designed. In this paper, the fabrication of a cloud chamber, experimental procedures and preliminary results of some experiments in the presence/absence of an external electric field will be presented.
2 The design and fabrication of the cloud chamber
The chamber has been designed and fabricated with an internal dimension of 1 ft \(\times \) 1 ft with aluminium walls inside the chamber. A compressor has been used to cool the inside of the chamber to –25°C from room temperature. Figure 1(a–b) shows the front and side views of the cloud chamber. An arrangement has been made to access and view the inside of the chamber through the front door with LED lighting inside the chamber. The temperature inside the chamber was directly measured with a platinum resistance thermometer (PT-100) inserted inside the chamber.
3 The experiment
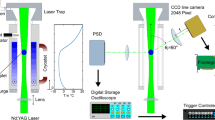
In the first experiment, drops of pure water (conductivity = 0.056 µ semen cm−1) with an approximate size of 1 mm were kept suspended in a Nylon wire. Pruppacher (1963) reported that water droplet with similar conductivity contains particles smaller than 0.01 µ in diameter. The drops are suspended in the Nylon wire using a syringe which produces drops of uniform size: approximately 1 mm. The experimental setup has been depicted in figure 2. The chamber was allowed to cool from room temperature to the temperature of −25°C. Another PT-100 was installed near the drops to detect the freezing of the drop. The PT-100 is calibrated to measure 0.1 V in every 1°C change in temperature. When the phase change happens, latent heat of fusion is released by the drops. A temperature controller connected to a local computer through a data logger is used to record the temperature rise at the freezing point. Figure 3 depicts a typical cooling curve record by the temperature controller. This temperature controller can measure temperature at a resolution of 0.1°C. The freezing point can be clearly detected with the rise in temperature. The relative humidity and aerosol concentration inside the chamber are kept as the ambient air at the beginning of the experiment. More than 100 samples are collected in this setup. It may be noted here that all the experiments are carried out with one drop at a time.
In the second experiment, the water drops are suspended as the first one. The drops are subjected to dc voltage in the range of 1–5 kV cm−1 using a dc power supply. Two plate electrodes made up of aluminium are used for the application of an electric field. It has also been ensured that no electric discharge takes place during the experiment as an electric discharge near the drop may create a mechanical disturbance to the drops. For this purpose, the two highly polished electrodes are kept parallel to each other, similar to Pruppacher (1963). In this setup, more than 40 samples are collected.
4 Results and discussion
In this section, we present some preliminary results of the experiment. Figure 4(a) shows a bar representation of the frequency distribution of the freezing temperature when the drop is allowed to freeze without any electrical influence. The drops are observed to freeze in the temperature ranging from −14° to −16°C. Approximately 36% drops are observed to freeze between −14° to −16°C. This result is consistent with the experiment of Abbas and Latham (1969).
Figure 4(b) depicts the frequency distribution of the freezing temperature when the drops are subjected to an electric field of magnitude ranging 2.5–5 kVcm−1. A shift in the distribution towards warmer temperature is evident from the figure. When the drops are subjected to an electric field, most of the drops freeze between −6° and −10°C. It may be noted here that as this result is derived from around 40 experiments, the most likely freezing temperature in the presence of an external electric field may vary with more samples.
Visual observations suggest that in the presence of an electric field, for a few of the drops, crystallization starts at the surface, while for others, the crystallization process is observed to initiate at the centre of the drops. The mode of freezing will be investigated with high-speed camera photography in future experiments. It may be noted here that, although all the experiments have been performed using distilled water drops, the surrounding environment of the cloud chamber remains largely uncontrolled. This may be one possible reason for the large variability in the observed freezing temperature in both the set of experiments. It is known that various aerosol particles such as mineral dust, soot, fungal spores, pollen and bacteria may also act as ice nuclei which may induce freezing (Hoose et al. 2010; Hazra 2013). These aerosol particles vary depending upon the surrounding environment. Hence, a larger variability in the freezing temperature of the drops can be expected depending on the surrounding environment.
The cause of the shift in freezing temperature in the presence of an electric field could not be ascertained at present. Pruppacher (1963) suggested that the movement of the water interface of deformed super-cooled water drops on a solid surface can cause freezing in the presence of an electric field. As explained above, all the experiments reported in this study are performed by keeping the drops suspended in a Nylon wire. During the freezing process, no deformation and movement of the drops in the Nylon wire are observed. Loeb (1963) suggested that the presence of a super-cooled solid surface (in this case, the Nylon thread) which can efficiently absorb the latent heat of fusion can facilitate the freezing of the drops. He suggests the essential requirement of free space for volume expansion during freezing. Loeb (1963) also suggested that an intense electric field can draw out fine filaments from the drops, which can initiate the freezing. The possible explanation of the current observation of electro-freezing may be the absorption of the latent heat of fusion by the Nylon wire where the drops are kept suspended during the experiments. The observed warmer freezing of the water drops in presence of an electric field may explain the presence of a larger concentration of ice crystals inside the strongly electrified clouds.
It is conceivable that if an electric field in a strongly electrified cloud which may go up to \(4\times {10}^{5}\) V m−1 (Winn et al. 1974) can induce freezing at a warmer temperature, it can potentially impact the dynamics of the storm by releasing the latent heat of freezing. Also, the electro-freezing process may act as a source of secondary ice production in the mixed-phase layer of the cloud which can further facilitate the electrification of the cloud. As the present experiments have been carried out by providing the drops with a solid surface (the Nylon wire), direct projection of these results to real clouds may not be linear in general. However, as suggested by Pruppacher (1963), such surfaces can be introduced into the clouds by artificial cloud seeding. The results presented in this paper may act as a convincing basis for further research of electrically induced crystallization of supercooled liquid water from the perspective of cloud electrification and consequent cloud microphysical and dynamical implications.
References
Abbas M A and Latham J 1969 The electrofreezing of supercooled water drops; J. Meteorol. Soc. Japan 47 65–74.
Anderson R J, Miller R C, Kassner J L and Hagen D E 1980 A study of homogeneous condensation-freezing nucleation of small water droplets in an expansion cloud chamber; J. Atmos. Sci. 37(11) 2508–2520.
Ausman E L and Brook M 1967 Distortion and disintegration of water drops in strong electric fields; J. Geophys. Res. 72 6131–6135.
Bhalwankar R V, Sathe A B and Kamra A K 2004 The evaporation of the charged and uncharged water drops suspended in a wind tunnel; Proc. Indian Acad. Sci. (Earth Planet. Sci.) 113 129–138.
Bhalwankar R V and Kamra A K 2007 A wind tunnel investigation of the deformation of water drops in the vertical and horizontal electric fields; J. Geophys. Res. 112 D10215.
Bruning E C, Rust W D, Macgorman D R, Biggerstaff M I and Schuur T J 2010 Formation of charge structures in a supercell; Mon. Wea. Rev. 138(10) 3740–3761.
Hazra A 2013 Role of mineral dust, soot, and bacteria in cloud and precipitation formation processes over Indian subcontinent using an atmospheric general circulation model; J. Atmos. Sol. Terr. Phys. 98 74–85.
Hoose C, Kristjánsson J E, Chen J P and Hazra A 2010 A classical-theory-based parameterization of heterogeneous ice nucleation by mineral dust, soot and biological particles in a global climate model; J. Atmos. Sci. 67(8) 2483–2503.
Dawson G A and Cardell G R 1973 Electrofreezing of supercooled waterdrops; J. Geophys. Res. 78(36) 8864–8866.
Kamra A K and Ahire D V 1989 Wind tunnel studies of the shape of charged and uncharged water drops in the absence or presence of an electric field; Atmos. Res. 23 117–134.
Lapshin V B, Yablolov M Y and Palei A A 2002 Vapor pressure over a charged drop; Russian J. Phys. Chem. 76 1727–1729.
Loeb L B 1963 A tentative explanation of the electrical field effect on the freezing of supercooled water drops; J. Geophys. Res. 68(15) 4475–4476.
Mandal G and Pradeep Kumar P 2002 A laboratory study of ice nucleation due to electrical discharge; Atmos. Res. 61(2) 115–123.
Morrison H, Thompson G and Tatarskii V 2009 Impact of cloud microphysics on the development of trailing stratiform precipitation in a simulated squall line: Comparison of one- and two-moment schemes; Mon. Wea. Rev. 137(3) 991–1007.
Nielsen J K, Maus C, Rzesanke D and Leisner T 2011 Charge induced stability of water droplets in subsaturated environment; Atmos. Chem. Phys. 11 2031–2037.
Rayleigh L 1879 The influence of electricity on colliding water drops; Proc. Roy. Soc. London 28 406–409.
Richards C N and Dawson G A 1971 The hydro-dynamic instability of water drops falling at terminal velocity in vertical electric fields; J. Geophys. Res. 75 3445–3455.
Pruppacher H R 1963 The effect of an external electric field on the supercooling of water drops; J. Geophys. Res. 68(15) 4463–4474.
Pruppacher H R and Klett J D 1996 Microphysics of Clouds and Precipitation (2nd edn); Springer.
Salt R W 1961 Effect of electrostatic field on freezing of supercooled water and insects; Science 133(3451) 458–459.
Schaefer V J 1968 The generation of large numbers of ice crystals in an electric field; J. Appl. Meteor. 7(3) 452–455.
Stolzenburg M, Rust W D and Marshall T C 1998 Electrical structure in thunderstorm convective regions. 2: Isolated storms; J. Geophys. Res. Atmos. 103(D12) 14079–14096.
Takahashi T 1978 Riming electrification as a charge generation mechanism in thunderstorms; J. Atmos. Sci. 35(8) 1536–1548.
Taylor G 1964 The disintegration of water drops in an electric field; Proc. Roy. Soc. London Ser. A280 383–397.
Winn W P, Schwede G W and Moore C B 1974 Measurements of electric fields in thunderclouds; J. Geophys. Res. 79 1761–1767.
Acknowledgement
IITM is funded by the Ministry of Earth Sciences, Government of India. We thank the Director, IITM for his continuous support and encouragement. We are grateful to the Editor, Associate Editor, and two anonymous reviewers for their critical and constructive comments throughout the review process which greatly improved the manuscript.
Author information
Authors and Affiliations
Contributions
The contributions of the authors are as follows. Dipjyoti Mudiar: Conceptualization, experiment, formal analysis, and writing-original draft. Sunil D Pawar: Conceptualization, experiment, writing-original draft, and supervision. Anupam Hazra: Writing-original draft and supervision. Abhijeet Gangane: Experiment. V Gopalakrishnan: Writing-original draft. D M Lal: Supervision. M K Srivastava: Writing – Review and editing, supervision.
Corresponding author
Additional information
Communicated by Kavirajan Rajendran
Rights and permissions
About this article
Cite this article
Mudiar, D., Pawar, S.D., Hazra, A. et al. A laboratory investigation of electrical influence on the freezing of water drops: A cloud physics perspective. J Earth Syst Sci 130, 222 (2021). https://doi.org/10.1007/s12040-021-01736-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12040-021-01736-6