Abstract
Biofilm forming and heavy metal resistant marine bacterial strain Pseudomonas chengduensis PPSS-4 was isolated from the contaminated marine sediment of Paradip Port, Odisha, India. The strain showed biofilm formation up to 100 mg/L of multi-metal [Pb(II), Cr(VI), and Cd(II)] supplementation in the culture medium. Scanning electron microscopy (SEM) showed aggregation of rod-shaped cells in the extracellular polymeric substance (EPS) matrix of biofilm. Confocal laser scanning microscopy (CLSM) exhibited a higher nucleic acid to the α-polysaccharide ratio in the biofilm, and the observed thickness was ~21 µm. The metal uptake potential of biofilm culture was higher than planktonic culture both in single and multi-metal solutions. FESEM-EDS analysis revealed the sequestration of multi-metals by bacterial cells and biofilm-EPS. FTIR analysis of bacterial EPS further ensured the interaction of functional groups such as –OH, –NH, and P=O with the metal ions. The maximum removal of Pb, Cr, and Cd by the bacterial biomass was observed at 37°C within 4 h of contact time at pH 6, and 4% salinity for Pb and Cr, and 6% salinity for Cd. The present study revealed that the marine bacterium P. chengduensis PPSS-4 can remove multi-metals, and this bacterium could be efficiently utilized for the remediation of heavy metals in the contaminated environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biofilm formation is a strategy developed by microbes to survive and adapt to adverse environmental conditions. Biofilm is the complex communities of microbes that are attached to a substratum and sheltered within a matrix of extracellular polymeric substances (EPS). Bacteria form biofilm in the marine environment to cope with the dynamic changes, such as variation in sea surface temperature, alternation in pH, imbalance of osmotic pressure, and desiccation (Dash et al. 2013). In the marine environment, colonization of bacterial cells occurs in the submerged substratum such as rock, ship, etc. Biofilm formation is regulated by quorum sensing (QS), a density-dependent cell-to-cell communication mechanism that provides enhanced assemblage of cells, higher nutrient availability, and increased tolerance of biofilm community to environmental stresses (Salta et al. 2013). QS also regulates the EPS synthesis, chemotaxis, horizontal gene transfer, and catabolic gene expression for the degradation of toxic compounds (Mangwani et al. 2016). Microbial biofilm has been applied for bioremediation of recalcitrant materials as biofilm provides greater tolerance to dynamic environmental changes, including toxic pollutants and several other abiotic factors like pH, temperature, and salinity.
Ports play a pivotal role in the global economy, providing over 70% by value of international trade through marine transport (UNCTAD 2015). Due to rigorous transportation activities, port sites face enhanced environmental pressure from heavy metal contamination, oil spills, ballast water, domestic waste, ship paint, and other pollutants. Besides, various anthropogenic activities, including mining, smelting, household waste, agriculture, and aquaculture discharges, are dumping different hazardous chemicals into the marine ecosystem. Compared to the various pollutants, heavy metals pollution in coastal zones is regarded as one of the major environmental problems because of their long-term persistence, non-degradability, multiple sources of pollution, and bioaccumulation in the food chain. Industrial activities have immensely enhanced the release and subsequent accumulation of several hazardous heavy metals in the natural environment, causing a severe threat to the health and productivity of the natural biome (Hosono et al. 2011).
Among various hazardous metals, lead (Pb), cadmium (Cd), and chromium (Cr) were reported as systemic toxicants that have adverse effects on several organs, even at lower concentrations (Tchounwou et al. 2012). Generally, Pb contamination occurs through anthropogenic activities such as, manufacturing and recycling of batteries, book printing, synthesis of pigments, lead-containing paints and pipes, etc. Long-term exposure to Pb affects nearly all organs in humans, mainly associated with blood disorders and chronic damage to the nervous system (Ara and Usmani 2015). The Cd contamination in the environment generally occurs due to the combustion of metal-containing ores, burning of fossil fuels, and electroplating industries (Rahimzadeh et al. 2017). The accumulation of Cd in the food chain causes several ailments like mutations and deletions in chromosomes (Joseph 2009), induces apoptosis and ROS generation (Rani et al. 2014), and cancer (Kellen et al. 2007). However, Cr pollution is caused due to several natural and human-made activities, specifically discharge of industrial effluents, mineralization of metals, irrigation of sewage water, and excessive application of chemically synthesized pesticides and fertilizers (Vimercati et al. 2017). Thus, it is necessary to establish effective measures to remove toxic heavy metals that are accumulated in the natural environment.
The conventional physico-chemical processes that have been employed for the treatment of metal pollutants are expensive for separating toxic metals from dilute solutions (Vullo et al. 2008; Muñoz et al. 2012) and cause environmental pollution due to the synthesis of secondary toxic chemical sludge (Barkat 2011). Hence, the application of environment-friendly, cost-effective, feasible cleaning methods with little or no residual by-products is of utmost importance (Das and Dash 2014).
Microbe–metal interaction has gained the most pursued area in recent years for heavy metal bioremediation among the various promising methods. In this context, the application of marine bacteria in removing heavy metal from metal-contaminated sites is more efficient for enhanced bioremediation (Dash et al. 2013). Besides, the application of bacterial biofilm in metal removal is advantageous because of their higher sorption efficiency and low production cost (Kurniawan and Yamamoto 2013). Generally, the heavy metals accumulated in the environment are positively charged, and these cationic toxic heavy metals can adsorb onto the negatively charged sites of biofilm by electrostatic attraction. Hence, biofilms of various microbes have been observed as potential adsorbents for heavy metals (Gupta and Diwan 2017). Additionally, biomass generated from bacterial origins is feasible and cost-effective and can be efficiently used for the biosorption of metal ions (Vijayaraghavan and Yun 2008). Thus, appropriate applications of living as well as non-living cells of bacteria under customized physico-chemical parameters, including temperature, pH, salinity, concentrations of biomass, and metal ions, make them suitable agents for heavy metal bioremediation. However, it can be hypothesized that the biofilm environment may provide a protective milieu for bacterial cells to thrive under multi-metal stress. EPS, the protective covering of biofilm, may add increased sequestration and immobilization of metal ions. Moreover, the optimization of environmental parameters may contribute to the enhanced remediation of heavy metals by the bacterial biomass from the metal-contaminated aqueous solution.
There are various studies on the utilization of bacterial biomass for the uptake of single metal ions, but a few studies have reported the use of bacterial biomass and biofilm-EPS in multi-metal removal. Therefore, this study was carried out for isolation and characterization of multi-metal tolerant biofilm forming bacterial strain from the polluted coastal environment for bioremediation of toxic metals like lead, chromium, and cadmium. The study also explored the mechanisms of metal uptake and the optimal conditions for maximum metal removal by the marine bacterium P. chengduensis PPSS-4.
2 Materials and methods
2.1 Isolation of bacterial strain and growth conditions
The sediment samples were collected from contaminated sites of Paradip Port, Odisha, India, and were aseptically transported to the laboratory. The salinity of the water collected from the sampling site was recorded as 35 ppt. For isolation of multi-metal resistant bacteria, Luria Bertani (LB) agar (HiMedia, India) plates were prepared by supplementing varying concentrations (10, 20, 30, 40, and 50 mg/L) of Pb(II) as Pb(NO3)2 (HiMedia, India), Cr(VI) as K2Cr2O7 (HiMedia, India) and Cd(II) as CdCl2 (Merck, India). Sediment samples were serially diluted, followed by spreading over multi-metal appended LB agar plates and were incubated at 37°C till the visible bacterial colonies appeared. The pure culture of the isolates was grown in the LB agar medium and screened for biofilm formation ability.
Biofilm development by the bacterium was examined by both qualitative and quantitative assay. The microtiter plate assay was carried out following Jordjevic et al. (2002) with slight modifications (Mangwani et al. 2014). Briefly, the bacterial culture was inoculated into the freshly prepared LB medium followed by incubation at 37°C for overnight in a shaker incubator up to the optical density of 0.5 (OD600). From this, 2 µl of the bacterial culture suspension was appended to a 96-well microtiter plate containing 200 µl of LB medium, with and without supplementation of different concentrations (25, 50, 100, and 150 ppm) of multi-metals. The microtiter plate was kept at 37°C for 48 h in the static state. Negative control was taken in the well as sterile LB medium without the addition of bacterial culture. Following incubation, the microtiter plate wells were washed by using 1×PBS to take away the unattached cells. Crystal violet solution of 0.2% was used for staining the biofilm. The visible thick ring formation at the air–water interface suggests that the strain is a good biofilm former. Further, the biofilm formed by the bacterium was destained with 95% ethanol and quantified in a multi-plate reader (BioBase, PR China) by taking the absorbance of destained ethanol. The biofilm forming ability of the strain was examined by comparing it with the cut-off value (ODC) of the negative control. The ODC can be calculated by the formula, ODC = average OD of negative control + (3 × standard deviations of negative control). Depending upon the ODC value, the strain can be designated as weak (ODC < OD ≤ 2 × ODC), moderate (2 × ODC < OD ≤ 4 × ODC), or strong (4 × ODC < OD) biofilm former (Stepanović et al. 2007).
2.2 Metal analysis and MICs of heavy metals
The heavy metals present in the collected sediment samples were checked by Atomic Absorption Spectrometer (AAnalystTM 200, Perkin-Elmer, USA). Prior to the analysis, the soil samples were acid digested by following the protocol of Edgell (1988). Minimum inhibitory concentrations (MIC) of the isolate towards Pb(II), Cr(VI), and Cd(II) were determined by micro-broth dilution technique (CLSI 2006) using the salts of Pb(NO3)2 (HiMedia, India), K2Cr2O7 (HiMedia, India) and CdCl2 (Merck, India) respectively. For this, in a 96 welled flat-bottom microtiter plate, 150 μL of Mueller Hinton broth (MHB) (Himedia, India) containing different concentrations of Pb(II) was taken in the 1st to the 10th well. The 11th column with 150 μL of MHB was taken as the positive control, and the 12th column with metal appended MHB as the negative control. 20 μL of overnight grown cultures of bacteria with optical density 0.5 (OD600) was inoculated in each well of the microtiter plate except the last well taken as the negative control. The microtiter plate was incubated at 37°C for 48 h. After incubation, absorbance was taken at 600 nm using a multi-plate reader (Biobase, PR China). A similar procedure was followed to determine MIC towards the other two metals, i.e., Cr(VI) and Cd(II). The lowest concentration of the metals completely inhibiting bacterial growth was designated as the MIC of the respective metals. Based on the result of biofilm formation and the metal tolerance, the bacterial strain P. chengduensis PPSS-4 was selected and identified by the partial sequence of the 16S rRNA gene. The nucleotide sequence of this strain was submitted to NCBI GenBank under the accession number MT661599.
2.3 Characterization of the bacterial biofilm
The surface morphology of P. chengduensis PPSS-4 cells was analyzed by Scanning Electron Microscope (FEI Quanta FEG 250 SEM, USA). For this, the bacterium culture was inoculated into the sterile LB medium and kept at 37°C for incubation. The OD of the bacterial culture was adjusted to 0.5 at 600 nm wavelength, and 40 µl of this bacterial suspension was added over cut glass slides (1 × 1 cm) submerged in 4 ml of LB medium in a 6-well plate. The cell morphology under multi-metal treated conditions was analyzed by adding 100 mg/L of all the metal to the sterile LB medium. The plate was incubated for 48 h in a static condition at 37°C. The glass slides were then thoroughly rinsed by using 1× PBS to remove the planktonic cells. Further, the cells attached over the glass slides were fixed by adding 2.5% glutaraldehyde (HiMedia, India) followed by incubation for 12 h at 4°C. The fixed cells were then dehydrated using a series of increasing ethanol gradient concentrations, i.e., 20%, 40%, 60%, 80%, and 100%, and allowed to dry in a desiccator. The glass slides containing biofilm samples were taken for platinum coating and visualized under SEM at 20000× magnification (Chakraborty and Das 2014).
The biofilm components of P. chengduensis PPSS-4 were visualized under Confocal laser scanning microscopy (CLSM). Different components of biofilm, i.e., cells and EPS, were observed using the stain 5 µmol l−1 Syto9 (Invitrogen, USA) and 100 µg ml−1 ConA Alexa Fluor 633 conjugate (Invitrogen, USA), respectively. Syto9 was used to stain the nucleic acid of bacterial cells, and ConA Alexa Fluor 633 conjugate was used to stain the α-polysaccharide of EPS. As the bacterial EPS is composed of different carbohydrates in a higher amount, ConA Alexa Fluor 633 conjugate was used to stain the cell-bound EPS. The biofilm architecture was then detected under the Leica TCS SP8 confocal system (Leica Microsystems, Germany). Biofilm images were analyzed by using ImageJ 1.52.
2.4 Analysis of metal uptake by bacterial cells and EPS through SEM-EDS analysis
SEM and EDS analysis were performed to analyze the uptake of heavy metals by the cells and EPS of P. chengduensis PPSS-4. In the case of cells, a colony of the bacterium was inoculated in LB broth medium and was allowed to grow at 37°C overnight. In a 6-well plate, 40 µl of this overnight grown bacterial suspension was appended over small glass slides (1 × 1 cm) submerged in 4 ml LB broth medium with and without supplementation of metals, i.e., Cr(VI), Pb(II), and Cd(II) at a concentration of 100 mg/L each. The plate was incubated at 37°C for 48 h at a static state. After 48 h, the glass slides from the six-well plates were removed and washed with 1× PBS to wash out the unattached cells, followed by fixation with 2.5% glutaraldehyde. Then the glass slides were kept at 4°C for 12 h. The fixed cells on the slides were dehydrated using increasing concentrations of ethanol gradients, i.e., 20%, 40%, 60%, 80%, and 100%, and were observed under Field Emission Scanning Electron Microscope (NOVA FEI450, Switzerland) at 10000× magnification and analyzed through Quantax EDS attachment (Bruker AXS Ltd., Coventry, UK) (Chakraborty and Das 2014).
In the case of EPS, overnight grown culture of P. chengduensis PPSS-4 was diluted 100 times with sterile LB medium. From this, 50 ml of the culture suspension was dispensed to two separate flasks containing sterile glass beads (2 mm diameter), one with supplementation of all the metals (100 mg/L each) and another without any metal. The flasks were then incubated in a static condition at 37°C for 48 h, followed by the removal of planktonic cells by washing with 1× PBS. Then, the disintegration of biofilm was done by vigorously vortexing the glass beads, and the collected samples were centrifuged at 6500 rpm for 10 min at 4°C. The supernatant was taken and filtered by 0.2 µm membrane filter. Then to the supernatant, a double volume of 90% chilled ethanol was added and kept at 4°C for EPS precipitation. The EPS were acquired by centrifugation at 12,000 rpm at 4°C for 10 min. The pellet was taken and dried at 60°C to remove ethanol. The collected EPS was lyophilized, followed by coating with gold and visualized under Field Emission Scanning Electron Microscope (NOVA FEI450, Switzerland) at 10000× magnification and analyzed through Quantax EDS attachment (Bruker AXS Ltd., UK).
2.5 Uptake of heavy metals by living cells in planktonic and biofilm mode
One ml of freshly grown bacterial culture with optical density 0.5 (OD600) was added into a flask containing 100 ml LB broth medium and glass beads. The flasks were incubated at 37°C under static conditions for 48 h. Free planktonic cells and the spent medium were cautiously decanted by washing the flasks gently with PBS. For each metal, 100 ml of LB medium was supplemented with 100 mg/L of Pb, Cr, and Cd separately and poured over the biofilm containing flask followed by incubation for 24 h at 37°C. Similarly, for multi-metal ion solution, 100 mg/L of all the metals were added to the 100 ml of LB broth and incubated at the same condition. After incubation, the biofilm cultures were vortexed, followed by centrifugation for 10 min at 12,000 rpm. The supernatant was taken and analyzed by AAS. To evaluate the multi-metal biosorption potential of planktonic cells, 1 ml of the overnight grown culture of bacterial strain was inoculated in 100 ml LB medium supplemented with 100 mg/L of each metal. The flasks were incubated in shaking condition at 120 rpm at 37°C for 24 h. After incubation, the bacterial cultures were separated by centrifugation at 12,000 rpm for 10 min. The supernatants were collected, and residual metals were quantified by AAS.
2.6 Functional groups of bacterial EPS by FTIR
The multi-metal treated EPS of the strain, extracted by exposing the bacterial biofilm to Pb(II), Cr(VI), and Cd(II) 100 mg/L each, and was characterized for the functional groups that are involved in the adsorption of heavy metals. The lyophilized EPS samples, both in the presence and absence of multi-metals, were pelletized with IR grade KBr powder (HiMedia, India) and were used for FTIR analysis. Then, FTIR spectra of untreated and metal-treated EPS were recorded within the wavenumber range of 400–4000 cm−1 using the FTIR spectrometer (Perkin-Elmer, USA).
2.7 Preparation of biosorbent
For the biosorption study, a single bacterial colony was inoculated into a 500 ml flask having 200 ml of LB broth medium and allowed to grow for 48 h in an aerobic condition at 37°C. Then the biomass was collected by centrifuging the culture medium at 7000 rpm for 15 min. The bacterial biomass was then rinsed twice with deionized water, and the cell pellet was air-dried and used as biosorbents.
2.8 Batch biosorption and optimization of environmental parameters
Biosorption of Pb(II), Cr(VI), and Cd(II) by the biomass of P. chengduensis PPSS-4 was optimized under several crucial environmental factors, including temperature, pH, and salinity. The effect of temperature was studied at 25°, 37°, and 45°C in a shaker incubator at 150 rpm. The residual heavy metal concentrations were analyzed for every 1 h interval until heavy metal removal attains a saturation point. The biomass of the bacterium was constant (2 g/L) for all the experiments, and the concentrations of each metal were fixed at 10 mg/L. The pH of the biosorption medium was maintained at 2, 4, 6, 8, and 10 by adding 0.1M HCl and 1M NaOH. The centrifuge tubes were kept at 37°C in a shaking condition at 150 rpm for 4 h, as the saturation of the biosorption process was observed after 4 h of contact time. The effect of salinity on the biosorption of metal ions was studied under different NaCl concentrations, i.e., 2%, 4%, 6%, 8%, and 10% at 37°C, pH 6, and 150 rpm for 4 h.
To determine the effect of temperature, pH, and salinity in biosorption, aliquots of each reaction were taken aseptically and filtered. The filtrates were analyzed for estimation of metal concentration using AAS. The heavy metal ion solutions without bacterial biomass were taken as control. The heavy metal removal efficiency of bacterial biomass was calculated from the following equation: % Removal = (C0 – Ce)/C0 × 100, where C0 and Ce are the initial and residual metal ion concentrations (mg/L) in the solution, respectively.
2.9 Statistical analysis
All the experiments were done in triplicates and were expressed as mean ± SD. The heavy metal ion solutions (10 mg/L) without bacterial biomass were taken as control to normalize the metal removal rate under all conditions. For all the experiments, P < 0.05 was considered a statistically significant value. Statistical analyses were carried out by Graphpad Prism version 7.00. Two-way ANOVA and Sidak’s multiple comparison test was carried out for metal uptake by living cells of the bacteria in biofilm and planktonic mode. A two-way ANOVA and Tukey’s multiple comparisons test was done to estimate the effect of temperature and contact time on biosorption of metal ions. One-way ANOVA and Tukey’s multiple comparisons test were done for pH and salinity-dependent biosorption by the bacterial biomass.
3 Results
3.1 Metal analysis and MICs of heavy metals
The molecular identification of the strain was carried out using 16S rRNA partial gene sequencing, and the strain was identified as P. chengduensis PPSS-4. The phylogenetic tree was constructed by the Neighbour-Joining method, and evolutionary distances were calculated using the Tamura-Nei model clustered together in the bootstrap test of 1000 resamplings (figure 1). The concentrations of heavy metals such as, Pb, Cr, and Cd in the sediment sample were given in table 1. The MICs of these metals against the isolate were found to be 2300, 2000, and 1300 mg/L, respectively.
Phylogenetic analysis of the biofilm-forming metal resistant bacterium Pseudomonas chengduensis PPSS-4 based on partial sequence of 16S ribosomal RNA (rRNA) gene using MEGA 6.0. The tree was constructed using the neighbour joining method. Numbers at nodes are bootstrap values (%) based on neighbour joining analysis of 1000 replicates.
3.2 Biofilm assay
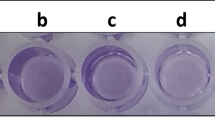
Biofilm formation by qualitative assay showed a thick ring formation in the air–water interface, which suggested that P. chengduensis PPSS-4 is a strong biofilm former. Biofilm formation in the presence of different concentrations of each metal (25, 50, 100, and 150 mg/L) in multi-metal supplemented media was also observed (figure 2a). The bacterium exhibited weak to strong biofilm formation up to 100 mg/L of each metal. However, there is no biofilm growth at 150 mg/L of the multi-metal treated condition. A similar result was observed in the case of quantitative biofilm assay (figure 2b).
Biofilm formation assay of P. chengduensis PPSS-4 grown at 37°C. (a) Qualitative biofilm assay in the presence of multi-metals (Cr + Pb + Cd) at different concentrations of each (25, 50, 100 and 150 mg/L), and (b) quantification of biofilm growth in the presence of multi-metals (Cr + Pb + Cd) at different concentrations of each (25, 50, 100 and 150 mg/L) by microtiter plate assay.
3.3 Characterization of bacterial biofilm
Scanning electron micrographs of untreated P. chengduensis PPSS-4 biofilm displayed that the smoothed surfaced rod-shaped cells inside the biofilm matrix were encapsulated within the dense EPS layer. However, the surface morphology of P. chengduensis PPSS-4 biofilm treated with multi-metals showed a bunch of cells loosely packed inside disintegrated EPS matrix (figure 3a). Confocal microscopy images of the P. chengduensis PPSS-4 biofilm showed the cells stained with syto9 and the EPS stained with conA Alexafluor 633 conjugate (figure 3b). ImageJ analysis exhibited a higher proportion of nucleic acids than α-polysaccharides in the P. chengduensis PPSS-4 biofilm (figure 3c). The thickness of the bacterial biofilm was ~21 µm.
Analysis of P. chengduensis PPSS-4 biofilm grown at 37°C after 48 h of incubation. (a) Scanning electron microscopy (I) control [LB medium] and (II) 100 mg/L each of Pb(II)+Cr(VI)+Cd(II) treated cells, (b) Confocal Laser Scanning Microscopy, biofilm stained with (I) Syto9, (II) ConA Alexaflour 633 conjugate, (III) merged images, and (IV) 3D surface plot and (c) raw integrated density of the amount of nucleic acid and α-polysaccharides calculated using ImageJ.
3.4 Analysis of metal uptake by bacterial cells and EPS through SEM-EDS analysis
The cell morphology of P. chengduensis PPSS-4 was smooth before the treatment of heavy metals (figure 4a, b). EDS elucidated the elemental compositions of the bacterial cells. This analysis confirmed the Pb, Cr, and Cd signals in their respective spectra of biomass loaded with multi-metal ions (figure 4c, d). However, no such signals were observed in the spectrum of pristine biomass. Further, in the case of bacterial EPS, no such signal appeared in the control condition (figure 4e, f), and the metal-loaded EPS showed salt-like deposition of heavy metals (figure 4g, h).
3.5 Uptake of heavy metals planktonic and biofilm mode of the living cells
The removal of Pb(II), Cr(VI), and Cd(II) by biofilm and free planktonic cells of the multi-metal resistant bacterium P. chengduensis PPSS-4 under the single and multi-metal system was shown in figure 5(a, b). In the biofilm mode, the bacterium showed significantly (P < 0.0001; two-way ANOVA and Sidak’s multiple comparison test) more removal of metal ions than the planktonic mode in single as well as ternary metal solutions. In single metal ion solutions, uptake of Pb(II), Cr(VI), and Cd(II) observed by planktonic cells of the bacterium was 84.73%, 85.35%, and 65.71%, respectively. The removal of the above metals by the biofilm cultures was 92.91%, 91.14%, and 74.5%, respectively. In ternary metal ions solution, the percentage of removal by planktonic and biofilm culture of the bacterium towards Pb(II), Cr(VI), and Cd(II) were (56.43 and 79.32), (80.17 and 86.73) and (13.09 and 46.36), respectively.
3.6 Analysis of functional groups of bacterial EPS
Various functional groups exist on the bacterial EPS promote binding with the heavy metals. The FTIR spectra of metal loaded and pristine EPS showed the occurrence of a number of functional groups such as phosphate, amino, and hydroxyl in the metal loaded EPS of P. chengduensis PPSS-4, which may be involved in the uptake of heavy metals (figure 6). A stretching of the band illustrating the vibration of the –OH groups of the carbohydrate and the –NH group of amide II were detected in the wavenumber range of 3424.79–1656.17 cm−1 in the pristine EPS, respectively. The absorption band assigned for the P=O stretching vibration of phosphate groups was detected at 1070.67 cm−1 in the pristine EPS extracted from the isolate. However, the interaction of EPS with Pb(II), Cr(VI), and Cd(II) resulted in significant shifting of hydroxyl, amine, and phosphate positions in the FTIR spectrum. Upon exposure with multi-metals, the peaks were shifted distinctly from 3424.79 to 3390.78 cm−1, from 1656.17 to 1648.88 cm−1, and from 1070.67 to 1063.38 cm−1 indicating the contribution of hydroxyl, amide, and phosphate groups in metal binding, respectively.
3.7 Influence of temperature on biosorption of heavy metals with respect to contact time
The effects of temperature on biosorption of Pb(II), Cr(VI), and Cd(II) were observed at three different temperatures such as 25°, 37°, and 45°C with respect to contact time for 5 h. The dose of the bacterial biomass for each reaction was constant, i.e., 2 g/L and the concentrations of metals taken were 10 mg/L. The residual metal concentrations for each metal were analyzed every hour by AAS (figure 7). The rate of uptake of metals increased significantly (P < 0.0001, two-way ANOVA and Tukey’s multiple comparisons test) with the increase in contact time with the biomass and the saturation point observed after 4 h of contact time for all the metals at different temperature. The increase in temperature from 25° to 37°C increased the uptake of metals, from 76.56% to 89.26%, 55.62% to 72.29%, and 56.5% to 72.76% for Pb(II), Cr(VI), and Cd(II), respectively. However, further increases in temperature from 37º to 45ºC declined the rate of removal of the same metal ions to 39.67%, 54.97%, and 64.53%, respectively.
3.8 Influence of pH on biosorption of heavy metals
pH is an important factor in solution chemistry. Hence, different pH ranges (2, 4, 6, 8, and 10) were taken into consideration for the uptake of Pb(II), Cr(VI), and Cd(II) at 37°C for 4 h of contact time using 2 g/L of biomass and 10 mg/L of metal concentration (figure 8). Removal efficiency by the bacterial biomass was significantly (P < 0.0001, one-way ANOVA, and Tukey’s multiple comparisons test) higher at pH 6.
3.9 Influence of salinity on biosorption of heavy metals
The effect of salinity on biosorption of Pb(II), Cr(VI), and Cd(II) was observed under different salt concentrations (2%, 4%, 6%, 8%, and 10%) at the optimum temperature (37°C) and pH (6) for 4 h of contact time (figure 9). The Pb(II) and Cr(VI) removal by the bacterial biomass was significantly (P < 0.0001, one-way ANOVA, and Tukey’s multiple comparisons test) higher in 4% NaCl solution than ≥ 6% NaCl solution. The maximum removal of Pb and Cr at 4% NaCl concentration was 65.85% and 79.65%, respectively. However, maximum Cd (69.03%) removal was observed in 6% NaCl solution, which was significantly (P < 0.0001, one-way ANOVA, and Tukey’s multiple comparisons test) ≥ 8% NaCl solution. The least removal of metal ions by P. chengduensis PPSS-4 biomass was observed at 10% NaCl solution, 12.53% for Pb, 28.23% for Cr, and 52.63% for Cd.
4 Discussion
Marine environments act as a natural sink for various types of pollutants, including toxic heavy metals. The pollution at coastal zones is increasing immensely by the gradual accumulation of these pollutants into the sea sites (Dharmendra et al. 2020). However, marine ecosystems accommodate a huge group of micro-organisms, and these micro-organisms have developed various resistant mechanisms to resist the changing conditions. Most of the bacterial species that have been isolated from these indigenous metal polluted environments showed greater tolerance to heavy metals (Matyar et al. 2008; Choińska-Pulit et al. 2018). The bacteria develop biofilm to protect the cells from different hostile conditions such as heavy metal stress (Meliani and Bensoltane 2016). Biofilm formation is an inherent property of bacteria in which the microbial population within the biofilm is enclosed within a chemically complex EPS matrix. EPS is enriched with several negatively charged functional groups such as carboxyl, hydroxyl, phosphate, etc., and acquires adsorptive as well as adhesive properties that facilitate binding with heavy metals via electrostatic interaction (Vimalnath and Subramanian 2018).
In comparison to other adsorbents used for the sorption of heavy metals, the biomass from the microbial origin acts as an efficient, cost-effective tool for utilization in bioremediation. Biomass derived from bacteria has been proved to be an efficient agent in removing toxic metals from the metal-polluted sites (Luo et al. 2014; Mohapatra et al. 2019). Besides, the microbial cells have developed diverse mechanisms to retaliate against metal stress conditions, which include biosorption to the cell wall, sequestration through the EPS matrix, precipitation, and internalization of the metal ions (Das et al. 2016). Additionally, environmental factors such as temperature, pH, and salinity can significantly influence the sequestration of heavy metals by the microbes (Rodríguez-Tirado et al. 2012). Thus, it is crucial to explore endemic microbes, which are inhabitants of metal-polluted sites to remediate toxic metal ions. In addition, the application of microbial sorbents in biofilm mode along with EPS can be advantageous to remediate heavy metals from the marine environment. Furthermore, the utilization of bacterial biomass by controlling environmental parameters can provide better results for metal bioremediation.
The present study reports the bioremediation efficiency of biofilm forming multi-metal tolerant bacterium P. chengduensis PPSS-4 towards Pb, Cr, and Cd under various environmental conditions. The bacterium was isolated from the sediment sample of Paradip port, Odisha coast, India. As the ports are repeatedly polluted with various metal-related activities, the area was chosen for sampling with the aim of obtaining metal tolerant marine bacterium. A number of studies have previously revealed that the bacteria isolated from the port sites showed more resistance to heavy metals (Zampieri et al. 2016; Mohapatra et al. 2017). MICs of Pb(II), Cr(VI), and Cd(II) were evaluated against the bacterial strain. The marine isolate showed tolerance towards high concentrations of each metal. In an earlier study, Devika et al. (2013) reported multi-metal resistance bacterial strains isolated from the Indian Ocean, and they found the maximum tolerance of isolates towards Pb(II) and Cd(II) were 800 and 150 mg/L, respectively. In the present study, the isolate exhibited resistance up to 2200 mg/L of Pb(II), 1900 mg/L of Cr(VI), and 1200 mg/L of Cd(II) (100 mg/L lower than the MIC values). Similarly, the isolate from Hurghada harbour, Red Sea, showed tolerance towards multiple metals (Moselhy et al. 2013). Several marine bacterial strains such as Pseudomonas aeruginosa JP-11 (Chakraborty and Das 2014), Bacillus arsenicus, Bacillus pumilus, Bacillus indicus, Bacillus clausii, Planococcus maritimus, and Staphylococcus pasteuri (Nithya et al. 2011), Pseudomonas aeruginosa, Brevibacterium iodinium, Bacillus pumilus and Alcaligenes faecalis (De et al. 2008) showing resistance to multi-metals have been reported. Thus, the isolated bacterial strain P. chengduensis PPSS-4 could be an ideal agent for bioremediation of Pb, Cr, and Cd under high metal stress conditions.
Biofilm is the colonization of sessile communities of bacteria on a substratum that gives bacteria an enhanced protective milieu by shielding them in the EPS matrix, which contributes to the enhanced tolerance of bacteria towards heavy metal stress. Chien et al. (2013) showed that the bacteria that reside in the biofilm environment are more protected from the deleterious effects of heavy metals. The metal sequestration efficiency of bacterial biofilm and EPS was reported earlier by Dash et al. (2017), where they have found that the biofilm-EPS of Bacillus cereus BW-201B was able to accumulate inorganic mercury via interaction with surface functional groups. Various studies also reported that the metal uptake potential of the bacterial biofilm cultures was greater than their planktonic counterpart (Pan et al. 2014; Andreasen et al. 2018; Völkel et al. 2018). In the present study, the isolate showed comparatively higher uptake of Pb(II), Cr(VI), and Cd(II) in biofilm mode as compared to the free planktonic cells both in single and multi-metal ion solution. The finding of this study was in accordance with Black et al. (2014), where the removal rate of Pb(II) by biofilm-forming bacteria was 83.7% and by suspended cells was 72.6%. However, removal of each metal from their single ion solution was observed greater than the ternary metal ion solution both in biofilm and planktonic mode of the bacterial culture in the present study. As the uptake of the single metal takes place preferentially over another metal ion, the presence of multiple metal ions in a system lowers the removal of each of the metal ions (Wang and Sun 2013; Sati et al. 2014). The biofilm culture of P. chengduensis PPSS-4 revealed a greater affinity for Pb, Cr, and Cd removal than the planktonic cells.
FTIR spectrum revealed the presence of hydroxyl, amino, and phosphate groups in the bacterial EPS that interact with Pb, Cr, and Cd. The band at 3424.79 cm−1 indicated the –OH group, 1656.17 cm−1 refers to the amide II group, and 1070.67 cm−1 represents the organic P–O group. However, the EPS treated with Pb(II), Cr(VI), and Cd(II) revealed the shifting of bands, which suggests that the hydroxyl, amide, and phosphate functional groups were associated with the metal removal. The involvement of various negatively charged functional groups such as hydroxyl, carboxyl, amine, phosphate, and sulphate from bacterial cells and EPS were shown to be effectively sequestering lead, chromium, and cadmium ions (Naik et al. 2012; Chakraborty and Das 2014; Yue et al. 2015; Kumari et al. 2017; Vimalnath and Subramanian 2018). This inherent property of microbes towards metallic elements provides a suitable approach for remediation of metal-polluted sites. The SEM-EDS analysis also showed the precipitation of metals over the bacterial cells and EPS, which confirmed the involvement of functional groups in the metal accumulation. Similar result was observed by Saranya et al. (2018) for bio-removal of multi-metals. The changes in the cell morphology of the bacteria after the treatment with Pb(II), Cr(VI), and Cd(II) suggested the protective mechanism shown by the bacterial strain against the metal stress. Such a change in cell morphology was reported earlier in the Acidiphilium symbioticum H8 strain treated with multiple metal ions (Chakravarty and Banerjee 2008). The EPS extracted from P. chengduensis PPSS-4 was enriched with various anionic functional groups with the affinity to bind or sequester positively charged heavy metal ions from the metal-contaminated solution.
In case of in-situ bioremediation of toxic heavy metals, microbes withstand varied environmental conditions. Hence, it is crucial to analyze their metal uptake efficiency under different environmental settings. The present study reports the utilization of P. chengduensis PPSS-4 biomass for the removal of Pb(II), Cr(VI), and Cd(II). Among the different temperature ranges taken for the biosorption experiment, the optimum temperature for maximum sorption of all the three metals was observed at 37°C. The effect of temperature on the biosorption process revealed that the removal rate of metal ions was enhanced as the temperature of the medium raised from 25° to 37°C, and a further increase in temperature resulted in the declined rate of metal uptake. The result was corroborating with the biosorption efficiency of other mesophilic bacteria (Okeke 2008; Kumar et al. 2009; Mubashar and Faisal 2012). According to Burnett et al. (2006), over different temperature ranges, the protonation response of bacterial cells may vary, which in turn alters the metal removal efficiency with changing temperature. The rise in temperature enhances the uptake of metals due to the activation of functional sites on the biomass. But, a further increase in temperature above the optimum condition resulted in the degradation of functional sites on the biosorbent (Zouboulis et al. 2004). The optimum temperature within a range of 25°–40°C is preferable for higher removal of metal ions, as temperature regulates the kinetic state of the reaction and surface activity of the solute (Uslu and Tanyol 2006; Congeevaram et al. 2007). Hence, at an optimized temperature, the biomass of P. chengduensis PPSS-4 can uptake Pb, Cr, and Cd with high efficiency.
The uptake rate of metal ions by P. chengduensis PPSS-4 biomass is very rapid during the initial 2 h and approached equilibrium after 4 h of contact time. Further, with the increase in contact time, uptake rate was decreased for all the metals. Similar adsorption was observed by Alteromonas macleodii, which showed maximum biosorption of Cd(II) within 5 h of contact time (Moselhy et al. 2013). Bacillus spp. has displayed maximum metal removal within a contact time of 5 h (Pun et al. 2013), and Pantoea sp. TEM18 showed more rapid biosorption of Cr(VI) and Cd(II) within an initial contact time of 5 min (Ozdemir et al. 2004). In addition, the Pantoea sp. TEM18 biomass exhibited more rapid removal in the initial stage of biosorption followed by a slow removal phase until it reaches equilibrium. A similar effect of contact time on biosorption of toxic heavy metal was observed by different researchers (Selatnia et al. 2004; Odokuma and Akponah 2010). The effect of contact time on metal biosorption depends upon the biomass used and the target metal ion and also varies from a few minutes to several hours.
The pH of the reaction medium is a crucial factor that influences the adsorption of heavy metals to the bacterial biomass. Uptake of Pb(II), Cr(VI), and Cd(II) was carried out at different pH (2, 4, 6, 8, and 10) at optimum temperature, i.e., at 37°C. The highest removals of all the above metals were observed at pH 6 compared to the other pH values. Similar result was observed by Halttunen et al. (2007), where they showed maximum removal of Pb(II) and Cd(II) at pH 6. The maximum removal of Cr(VI) at pH 6 was obtained by P. aeruginosa biomass (Sethuraman and Balasubramanian 2010). However, in the present study, pH-dependent biosorption showed relatively less removal of metals in the reaction medium with a pH below 6. The observed reduced sorption rate at lower pH values could be due to the competition among hydrogen ions and the heavy metals for binding sites of the functional groups on the biosorbents (Sahmoune 2018). Besides, the high concentration of H+ ions at lower pH conditions protonates the functional binding sites of the biosorbents, and the reduction of the negative charge intensity on the biomass results in the inhibition of binding with the heavy metals (Bai et al. 2013). The increase in pH of the solution saturates the H+ ions in the reaction medium. As a result, surface negative charges of the biomass increase that make it more efficient in binding with the positively charged metal ions (Wierzba and Latała 2010). The observed uptake rate of Pb(II), Cr(VI), and Cd(II) by the P. chengduensis PPSS-4 biomass at pH 8 and 10 were relatively lower than the pH 6. Further increase in the solution above pH 6 reduced the metal removal rate by the bacterial biomass. Similar to the current finding, Oves et al. (2013) reported the biosorption behaviour of Bacillus thuringiensis strain OSM29 biomass for Pb, Cr, and Cd removal and reported that increasing pH of the medium ≥ 8 decreased the sorption rate. The reduction in metal uptake occurs at a higher alkaline pH medium due to decreased metal solubility in this condition (Puranik and Paknikar 1999). At high pH, the solution may result in deformation of the binding sites of the biosorbent that causes inhibition of metal uptake (Babák et al. 2013). The medium pH of the solution favours maximum sorption of the heavy metals by contributing metal solubility and activation states of the functional groups (Kumar et al. 2009). The P. chengduensis PPSS-4 biomass showed substantial biosorption of Pb, Cr, and Cd at all the tested pH medium. However, optimization of the pH in the reaction medium increased the rate of uptake significantly.
The salinity of the reaction medium exhibits an essential factor in the biosorption of metal ions. Biosorption of Pb(II), Cr(VI), and Cd(II) was studied at different salinity levels (2, 4, 6, 8, and 10% of NaCl) at 37ºC and pH 6. The maximum removal of Pb and Cr was observed at 4% of NaCl. However, there was no significant variation observed in the removal of Pb and Cr in 2% and 4% NaCl supplemented medium. The rate of biosorption for all metals was significantly reduced after 6% NaCl. Similar result for the Pb removal by Bacillus xiamenensis sp. PbRPSD202 was observed at 4% NaCl medium with maximum uptake of 89.32% (Mohapatra et al. 2019). Okeke (2008) also showed 76.5% of Cr removal at 5% NaCl concentration by Exiguobacterium sp. GS1 and the uptake efficiency were decreased with the further increase in salt concentrations. The removal of Cd was also not affected by 2–6% of NaCl. However, the greater removal of Cd was observed at 6% NaCl. Zhou et al. (2013) also showed more than 90% removal of Cd up to 5% NaCl by Pseudoalteromonas sp. SCSE709-6. In the present study, the rate of metal uptake reduced significantly at a salinity level ≥ 8%. The observed declined uptake rate at higher saline conditions could be due to the competition among Na+ and the heavy metals for binding sites of the functional groups on the biosorbents. However, Na+ competes with the metal cations at higher saline medium and binds with the maximum functional sites on the biomass, while at lower saline medium Na+ is less competitive and allows binding of the metals to the biosorbent surface (Green-Ruiz et al. 2008). The bacterial biomass was also able to remove Cd (52.63%), Cr (28.23%), and Pb (12.53%) at a very high concentration of NaCl, i.e., 10%. Hence, the biomass originated from P. chengduensis PPSS-4 can act as a potential adsorbent to remove multi-metal ions in low to high saline medium.
The marine isolate P. chengduensis PPSS-4 showed high tolerance against Pb, Cr, and Cd with MICs of 2300, 2000 and 1300 mg/L, respectively. The bacterium was able to form biofilm in the multi-metal appended medium. Both the bacterial biomass and EPS have shown Pb, Cr, and Cd sequestration ability. The biofilm culture of the bacteria more efficiently removed the metals compared to the planktonic cells. The involvement of functional binding sites in the EPS, i.e., –OH, –NH, and P=O towards metal ion binding, was confirmed. The environmental parameters such as temperature, pH, and salinity exhibited significant influence on Pb, Cr, and Cd removal by P. chengduensis PPSS-4 biomass, with greater uptake efficiency at 37°C, pH 6 and salinity 4% for Pb and Cr and 6% for Cd. Hence, the strain P. chengduensis PPSS-4 could be suitably applied to remove Pb, Cr, and Cd under varied environmental conditions.
5 Conclusion
The marine bacterium P. chengduensis PPSS-4 was a strong biofilm former and showed tolerance to the high concentration of Pb, Cr, and Cd. The strain has been found to be a competent agent for the removal of toxic metal ions. The uptake behaviour of bacterial cells in biofilm mode is greater than the planktonic mode for all three metals. The presence of prominent functional groups on the EPS helps in binding to the positively charged metal ions. The dried biomass of the bacteria showed higher removal of the toxic metals at 37°C, pH 6, 4% salinity for Pb and Cr and 6% for Cd within 4 h of contact time. Thus, the biofilm-EPS and biomass of bacterium P. chengduensis PPSS-4 have exhibited great potential for multi-metal removal in diverse conditions and could be applied as an eco-friendly, efficient, and cost-effective substitute over conventional physico-chemical approaches for heavy metal remediation.
References
Andreasen R, Li Y, Rehman Y, Ahmed M, Meyer R L and Sabri A N 2018 Prospective role of indigenous Exiguobacterium profundum PT 2 in arsenic biotransformation and biosorption by planktonic cultures and biofilms; J. Appl. Microbiol. 124(2) 431–443.
Ara A and Usmani J A 2015 Lead toxicity: A review; Interdisc. Toxicol. 8(2) 55–64.
Babák L, Šupinova P, Zichova M, Burdychova R and Vítová E 2013 Biosorption of Cu, Zn and Pb by thermophilic bacteria – effect of biomass concentration on biosorption capacity; Acta Univ; Agric. et Silvic. Mendelianae Brun. 60(5) 9–18.
Bai H, Han Y, Kang Y and Sun J 2013 Removal of Cu (II) and Fe (III) from aqueous solutions by dead sulfate reducing bacteria; Front. Chem. Sci. Eng. 7(2) 177–184.
Barakat M A 2011 New trends in removing heavy metals from industrial wastewater; Arab. J. Chem. 4(4) 361–377.
Black R, Sartaj M, Mohammadian A and Qiblawey H A 2014 Biosorption of Pb and Cu using fixed and suspended bacteria; J. Environ. Chem. Eng. 2(3) 1663–1671.
Burnett P G G, Daughney C J and Peak D 2006 Cd adsorption onto Anoxybacillus flavithermus: Surface complexation modeling and spectroscopic investigations; Geochim. Cosmochim. Acta 70(21) 5253–5269.
Chakraborty J and Das S 2014 Characterization and cadmium-resistant gene expression of biofilm-forming marine bacterium Pseudomonas aeruginosa JP-11; Environ. Sci. Pollut. Res. 21(24) 14,188–14,201.
Chakravarty R and Banerjee P C 2008 Morphological changes in an acidophilic bacterium induced by heavy metals; Extremophiles 12(2) 279–284.
Chien C C, Lin B C and Wu C H 2013 Biofilm formation and heavy metal resistance by an environmental Pseudomonas sp; Biochem. Eng. J. 78 132–137.
Choińska-Pulit A, Sobolczyk-Bednarek J and Łaba W 2018 Optimization of copper, lead and cadmium biosorption onto newly isolated bacterium using a Box-Behnken design; Ecotoxicol. Environ. Saf. 149 275–283.
CLSI W 2006 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; 7th edn, Clinical and Laboratory Standards Institute Approve Standard M7–A7; CLSI, PA, USA.
Congeevaram S, Dhanarani S, Park J, Dexilin M and Thamaraiselvi K 2007 Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates; J. Hazard. Mater. 146(1–2) 270–277.
Das S and Dash H R 2014 Microbial bioremediation: A potential tool for restoration of contaminated areas; In: Microbial Biodegradation and Bioremediation (ed.) S Das, Elsevier. USA, pp. 1–21.
Das S, Dash H R and Chakraborty J 2016 Genetic basis and importance of metal resistant genes in bacteria for bioremediation of contaminated environments with toxic metal pollutants; Appl. Microbiol. Biotechnol. 100(7) 2967–2984.
Dash H R, Basu S and Das S 2017 Evidence of mercury trapping in biofilm-EPS and mer operon-based volatilization of inorganic mercury in a marine bacterium Bacillus cereus BW-201B; Arch. Microbiol. 199(3) 445–455.
Dash H R, Mangwani N, Chakraborty J, Kumari S and Das S 2013 Marine bacteria: Potential candidates for enhanced bioremediation; Appl. Microbiol. Biotechnol. 97(2) 561–571.
De J, Ramaiah N and Vardanyan L 2008 Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury; Mar. Biotechnol. 10(4) 471–477.
Devika L, Rajaram R and Mathivanan K 2013 Multiple heavy metal and antibiotic tolerance bacteria isolated from equatorial Indian Ocean; Int. J. Microbiol. Res. 4(3) 212–218.
Dharmendra S, Kumar M R, Chinmayee A, Ranjan S D and Ranjan P C 2020 Assessment of marine sediment contamination and detection of their potential sources at Paradip port, East Coast of India; Res. J. Chem. Environ. 24 1–6.
Edgell K 1988 USEPA method study 37: SW-846 method 3050 acid digestion of sediments, sludges, and soils (EPA Contract No. 68-03-3254); Environmental Monitoring Systems Lab., Cincinnati, OH.
Green-Ruiz C, Rodriguez-Tirado V and Gomez-Gil B 2008 Cadmium and zinc removal from aqueous solutions by Bacillus jeotgali: pH, salinity and temperature effects; Bioresour. Technol. 99(9) 3864–3870.
Gupta P and Diwan B 2017 Bacterial exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies; Biotechnol. Rep. 13 58–71.
Halttunen T, Salminen S and Tahvonen R 2007 Rapid removal of lead and cadmium from water by specific lactic acid bacteria; Int. J. Food Microbiol. 114(1) 30–35.
Hosono T, Su C C, Delinom R, Umezawa Y, Toyota T, Kaneko S and Taniguchi M 2011 Decline in heavy metal contamination in marine sediments in Jakarta Bay, Indonesia due to increasing environmental regulations; Estuar. Coast. Shelf. Sci. 92(2) 297–306.
Jordjevic D, Wiedmann M and McLandsborough L A 2002 Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation; Appl. Environ. Microbiol. 68 2950–2958.
Joseph P 2009 Mechanisms of cadmium carcinogenesis; Toxicol. Appl. Pharm. 238(3) 272–279.
Kellen E, Zeegers M P, Den Hond E and Buntinx F 2007 Blood cadmium may be associated with bladder carcinogenesis: The Belgian case-control study on bladder cancer; Cancer Detect. Prev. 31(1) 77–82.
Kumar R, Singh R, Kumar N, Bishnoi K and Bishnoi N R 2009 Response surface methodology approach for optimization of biosorption process for removal of Cr (VI), Ni (II) and Zn (II) ions by immobilized bacterial biomass sp. Bacillus brevis; Chem. Eng. J. 146(3) 401–407.
Kumari S, Mangwani N and Das S 2017 Interaction of Pb (II) and biofilm associated extracellular polymeric substances of a marine bacterium Pseudomonas pseudoalcaligenes NP103; Spectrochim; Acta A. 173 655–665.
Kurniawan A and Yamamoto T 2013 Biofilm polymer for biosorption of pollutant ions; Procedia Environ; Sci. 17 179–187.
Luo S, Li X, Chen L, Chen J, Wan Y and Liu C 2014 Layer-by-layer strategy for adsorption capacity fattening of endophytic bacterial biomass for highly effective removal of heavy metals; Chem. Eng. J. 239 312–321.
Mangwani N, Kumari S and Das S 2016 Bacterial biofilms and quorum sensing: Fidelity in bioremediation technology; Biotechnol. Genet. Eng. 32(1–2) 43–73.
Mangwani N, Shukla S K, Kumari S, Rao T S and Das S 2014 Characterization of Stenotrophomonas acidaminiphila NCW-702 biofilm for implication in the degradation of polycyclic aromatic hydrocarbons; J. Appl. Microbiol. 117(4) 1012–1024.
Matyar F, Kaya A and Dinçer S 2008 Antibacterial agents and heavy metal resistance in Gram-negative bacteria isolated from seawater, shrimp and sediment in Iskenderun Bay, Turkey; Sci. Total Environ. 407(1) 279–285.
Meliani A and Bensoltane A 2016 Biofilm-mediated heavy metals bioremediation in PGPR Pseudomonas; J. Bioremediat. Biodegrad. 7(370) 2.
Mohapatra R K, Parhi P K, Pandey S, Bindhani B K, Thatoi H and Panda C R 2019 Active and passive biosorption of Pb (II) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: Kinetics and isotherm studies; J. Environ. Manag. 247 121–134.
Mohapatra R K, Parhi P K, Thatoi H and Panda C R 2017 Bioreduction of hexavalent chromium by Exiguobacterium indicum strain MW1 isolated from marine water of Paradip Port, Odisha, India; Chem. Ecol. 33(2) 114–130.
Moselhy K M, Shaaban M T, Ibrahim H A and Abdel-Mongy A S 2013 Biosorption of cadmium by the multiple-metal resistant marine bacterium Alteromonas macleodii ASC1 isolated from Hurghada Harbour, Red Sea; Arch. Sci. 66(2) 259–272.
Mubashar K and Faisal M 2012 Uptake of toxic Cr(VI) by biomass of exo-polysaccharides producing bacterial strains; Afr. J. Microbiol. Res. 6(13) 3329–3336.
Muñoz A J, Ruiz E, Abriouel H, Gálvez A, Ezzouhri L, Lairini K and Espínola F 2012 Heavy metal tolerance of microorganisms isolated from wastewaters: Identification and evaluation of its potential for biosorption; Chem. Eng. J. 210 325–332.
Naik M M, Pandey A and Dubey S K 2012 Biological characterization of lead-enhanced exopolysaccharide produced by a lead resistant Enterobacter cloacae strain P2B; Biodegradation 23(5) 775–783.
Nithya C, Gnanalakshmi B and Pandian S K 2011 Assessment and characterization of heavy metal resistance in Palk Bay sediment bacteria; Mar. Environ. Res. 71(4) 283–294.
Odokuma L O and Akponah E 2010 Effect of concentration and contact time on heavy metal uptake by three bacterial isolates; J. Environ. Chem. Ecotoxicol. 2(6) 84–97.
Okeke B C 2008 Bioremoval of hexavalent chromium from water by a salt tolerant bacterium, Exiguobacterium sp. GS1; J. Ind. Microbiol. Biot. 35(12) 1571–1579.
Oves M, Khan M S and Zaidi A 2013 Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil; Saudi J. Biol. Sci. 20(2) 121–129.
Ozdemir G, Ceyhan N, Ozturk T, Akirmak F and Cosar T 2004 Biosorption of chromium (VI), cadmium (II) and copper (II) by Pantoea sp. TEM18; Chem. Eng. J. 102(3) 249–253.
Pan X, Liu Z, Chen Z, Cheng Y, Pan D, Shao J and Guan X 2014 Investigation of Cr (VI) reduction and Cr (III) immobilization mechanism by planktonic cells and biofilms of Bacillus subtilis ATCC-6633; Water Res. 55 21–29.
Pun R, Raut P and Pant B R 2013 Removal of chromium (VI) from leachate using bacterial biomass; Sci. World. 11(11) 63–65.
Puranik P R and Paknikar K M 1999 Biosorption of lead, cadmium, and zinc by Citrobacter strain MCM B-181: Characterization Studies; Biotechnol. Prog. 15(2) 228–237.
Rahimzadeh M R, Rahimzadeh M R, Kazemi S and Moghadamnia A A 2017 Cadmium toxicity and treatment: An update; Caspian J. Intern. Med. 8(3) 135.
Rani A, Kumar A, Lal A and Pant M 2014 Cellular mechanisms of cadmium-induced toxicity: A review; Int. J. Environ. Health Res. 24(4) 378–399.
Rodríguez-Tirado V, Green-Ruiz C and Gómez-Gil B 2012 Cu and Pb biosorption on Bacillus thioparans strain U3 in aqueous solution: Kinetic and equilibrium studies; Chem. Eng. J. 181 352–359.
Sahmoune M N 2018 Performance of Streptomyces rimosus biomass in biosorption of heavy metals from aqueous solutions; Microchem. J. 141 87–95.
Salta M, Wharton J A, Blache Y, Stokes K R and Briand J F 2013 Marine biofilms on artificial surfaces: Structure and dynamics; Environ. Microbiol. 15(11) 2879–2893.
Saranya K, Sundaramanickam A, Shekhar S, Meena M, Sathishkumar R S and Balasubramanian T 2018 Biosorption of multi-heavy metals by coral associated phosphate solubilising bacteria Cronobacter muytjensii KSCAS2; J. Environ. Manage. 222 396–401.
Sati M, Verma M and Rai J P N 2014 Biosorption of heavy metals from single and multimetal solutions by free and immobilized cells of Bacillus megaterium [J]; Int. J. Adv. Manuf. Tech. 2(6) 923–934.
Selatnia A, Boukazoula A, Kechid N, Bakhti M Z, Chergui A and Kerchich Y 2004 Biosorption of lead (II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass; Biochem. Eng. J. 19(2) 127–135.
Sethuraman P and Balasubramanian N 2010 Removal of Cr (VI) from aqueous solution using Bacillus subtilis, Pseudomonas aeruginosa and Enterobacter cloacae; Int. J. Eng. Sci. Technol. 2(6) 1811–1825.
Stepanović S, Vuković D, Hola V, Bonaventura G D, Djukić S, Ćirković I and Ruzicka F 2007 Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci; Apmis. 115(8) 891–899.
Tchounwou P B, Yedjou C G, Patlolla A K and Sutton D J 2012 Heavy metal toxicity and the environment; In: Molecular, Clinical and Environmental Toxicology (ed.) Andreas L, Springer, pp. 133–164.
UNCTAD 2015 Review of Maritime Transport; In: UNCTAD/RMT/2015 Report, New York and Geneva.
Uslu G and Tanyol M 2006 Equilibrium and thermodynamic parameters of single and binary mixture biosorption of lead (II) and copper (II) ions onto Pseudomonas putida: Effect of temperature; J. Hazard. Mater. 135(1–3) 87–93.
Vijayaraghavan K and Yun Y S 2008 Bacterial biosorbents and biosorption; Biotechnol. Adv. 26(3) 266–291.
Vimalnath S and Subramanian S 2018 Studies on the biosorption of Pb (II) ions from aqueous solution using extracellular polymeric substances (EPS) of Pseudomonas aeruginosa; Colloid. Surface. B. 172 60–67.
Vimercati L, Gatti M F, Gagliardi T, Cuccaro F, Maria L D, Caputi A and Baldassarre A 2017 Environmental exposure to arsenic and chromium in an industrial area; Environ. Sci. Pollut. Res. 24 11,528–11,535.
Völkel S, Fröls S and Pfeifer F 2018 Heavy metal ion stress on Halobacterium salinarum R1 planktonic cells and biofilms; Front. Microbiol. 9 3157.
Vullo D L, Ceretti H M, Daniel M A, Ramírez S A and Zalts A 2008 Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E; Bioresour. Technol. 99(13) 5574–5581.
Wang T and Sun H 2013 Biosorption of heavy metals from aqueous solution by UV-mutant Bacillus subtilis; Environ. Sci. Pollut. Res. 20(10) 7450–7463.
Wierzba S and Latała A 2010 Biosorption lead (II) and nikel (II) from an aqueous solution by bacterial biomass; Pol. J. Chem. Technol. 12(3) 72–78.
Yue Z B, Li Q, Li C C, Chen T H and Wang J 2015 Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria; Bioresour. Technol. 194 399–402.
Zampieri B D B, Pinto A B, Schultz L, de Oliveira M A and de Oliveira A J F C 2016 Diversity and distribution of heavy metal-resistant bacteria in polluted sediments of the Araça Bay, São Sebastião (SP), and the relationship between heavy metals and organic matter concentrations; Microb. Ecol. 72(3) 582–594.
Zhou W, Ma Y, Zhou J and Zhang Y 2013 Bio-removal of cadmium by growing deep-sea bacterium Pseudoalteromonas sp. SCSE709-6; Extremophiles 17(5) 723–731.
Zouboulis A I, Loukidou M X and Matis K A 2004 Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils; Process Biochem. 39(8) 909–916.
Acknowledgements
The authors acknowledge NIT Rourkela, Odisha, for providing the research facility. MP acknowledge the Department of Science & Technology, Govt. of India, for the INSPIRE Award (No. DST/INSPIRE Fellowship/2017/IF170195) for the doctoral study.
Author information
Authors and Affiliations
Contributions
MP executed the experiments and drafted the manuscript. SD conducted the field visit for sampling, designed the research, secured internal funding, supervised the experiments and finalized the manuscript.
Corresponding author
Additional information
Communicated by Maripi Dileep
This article is part of the topical collection: Advances in Coastal Research.
Rights and permissions
About this article
Cite this article
Priyadarshanee, M., Das, S. Bioremediation potential of biofilm forming multi-metal resistant marine bacterium Pseudomonas chengduensis PPSS-4 isolated from contaminated site of Paradip Port, Odisha. J Earth Syst Sci 130, 125 (2021). https://doi.org/10.1007/s12040-021-01627-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12040-021-01627-w