This article is dedicated to the memory of Mozafar Rabiei.
Abstract
In this study, the surface of silica-coated magnetic nanoparticles (Fe3O4@SiO2) were successfully functionalized by an organic ligand of 1, 4-Diazabicyclo[2.2.2]octane (DABCO)-sulfonic acid and used as a highly efficient catalyst for the synthesis of 4-aryl-NH-1, 2, 3-triazoles from the benzyl alcohol derivatives, nitromethane and sodium azide in ethanol. Furthermore, this catalyst could be recovered and reused five times without noticeable loss of activity.
Graphic Abstract
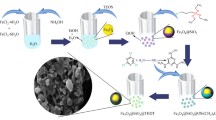
Fe3O4@SiO2@TCT-DABCO-SO3H nanoparticles were successfully synthesized and used as a novel, recyclable, efficient and heterogeneous catalyst for the synthesis of 4-aryl-NH-1, 2, 3-triazoles.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
More recently, 1, 2, 3-triazoles have attracted much attention, because of their significance of biological activities1 like anticancer, antibacterial, anti-HIV, antiallergic and other activities.2 The usual method to synthesize 1, 2, 3-triazoles is the cycloaddition reaction of an azide and an alkyne by catalyzed Cu(I) and Cu(II) salts (Huisgen reaction).3 Only a few methods can be used to synthesize N-unsubstituted 4-aryl-1, 2, 3-triazoles such as copper (I)-mediated azide-alkyne cycloadditions,4 reactions between alkenyl bromides and sodium azide,5 1,3-dipolar cycloadditions of nitro olefins and sodium azide,6 and also (E)-β-aryl vinyl bromides and sodium azide, under Pd2(dba)3 as a catalyst.7 Also, this reaction was performed in a toxic solvent.8 Therefore, the development of synthesizing N-unsubstituted 4-aryl-1, 2, 3-triazoles is still desirable. Nevertheless, the toxicity of transition metal such as copper sometimes is limited in biological and medical approaches.9,10
Lately, with increasing environmental concerns, magnetic nanoparticles have become a valuable group of heterogeneous catalysts. Also, separation of magnetic nanoparticles is simple and an attractive alternative to filtration as it doesn’t allow to lose catalyst activity and improves reusability. Recyclable magnetic nanoparticles have been made for using in and has attracted much interest across various reactions11,12,13,–14 as an improbable impact on the improvement of sustainable methods and provided the requirements meant for safe catalysis.
Therefore, our research groups tried to explain a method to achieve 4-aryl-NH-1, 2, 3-triazoles starting from benzyl alcohols, nitromethane and sodium azide with an efficient magnetic acid catalyst by a functionalized group of SO3H on the surface of silica-coated magnetite catalytic as Fe3O4@SiO2@TCT-DABCO-SO3H. We attempted to synthesize the above-mentioned catalyst under sulfonation. The conditions which have improved product yields also have several benefits such as (1) easy accessibility; (2) economical; (3) applicability. We used benzyl alcohols instead of benzaldehyde. Due to this, the catalyst helps the oxidation process of benzyl alcohols in the presence of hydrogen peroxide to perform tandem reaction through a simpler and economical way. Also, the direct synthesis 4-aryl-NH-1, 2, 3-triazoles from alcohols using functionalized heterogeneous catalysts has seldom been investigated. The attributes of solvent that extremely affects catalysis include polarity or dipolarity, hydrogen-bond donating potency and hydrogen-bond accepting potency. The catalyst activity is extremely affected by these parameters and provides the correct solvent for a catalytic reaction, or deciding how a solvent influence is significant in the reaction.15 Furthermore, volatile nature, much toxicity of solvents are becoming a highlighted subject which attracted chemist attention to the use of non-toxic solvents. Hence, our researcher teams decided to present environment-friendly and polarity solvent.16 Ethanol is known as a desirable green solvent because it is available as fermenting renewable sources, including sugars, starches, and lignocellulosic.17 According to the above mentioned, we decided to use ethanol as a safe, economical solvent to be able to diminish the use of a toxic solvent and also Fe3O4@SiO2@TCT-DABCO-SO3H nanoparticle was used as an organocatalyst for the synthesis of 4-aryl-NH-1, 2, 3-triazoles in ethanol as a solvent with good yields.
2 Experimental
2.1 Synthesis of catalyst
The synthesis of Fe3O4@SiO2@TCT-DABCO-SO3H is depicted in Scheme 1. First, Fe3O4 and Fe3O4@SiO2 were prepared according to the procedure previously reported.18 1 g of Fe3O4@SiO2, 1 mL 3-aminopropyltrimethoxysilane, 50 mL toluene were mixed and heated at 60 °C for 18 h. The resulting product was magnetically collected by an external magnet and was washed with toluene (40 mL) and methanol (30 mL) three times and dried at 60 °C in an oven. In the following, 1 g of Fe3O4@SiO2@(CH2)3NH2 10 mmol of Cyanuric chloride, 2 mL diisopropylethylamine, and 50 mL tetrahydrofuran were mixed and stirred at 0 °C for 4 h. Afterwards, the particles were washed with tetrahydrofuran (40 mL) and dried at room temperature under vacuum.
In the next step, 1 g of magnetic nanoparticle and 15 mmol of DABCO was added to 50 mL acetonitrile and stirred at reflux for 18 h. After that, the resulting product was washed with methanol (40 mL) and acetonitrile (40 mL) and dried in the oven at 60 °C. Finally, 2 mL of chlorosulfonic acid was added dropwise to a mixture of 1 g of Fe3O4@SiO2@TCT-DABCO in 50 mL dichloromethane and stirred at room temperature for 6 h. After that the resulting product was washed with dichloromethane (40 mL) and dried in the oven at 60 °C.
2.2 Characterization
All the chemical reagents used in this experiment were commercially available; Fourier-transform infrared (FT-IR) spectra were recorded using a Nicolet IR100 instrument in the region 400–4000 cm−1 with spectroscopic grade KBr. The 1H NMR (500 MHz) spectra were obtained by using a Bruker Advance DPX-500NMR spectrometer. Field scanning electron microscopy (FE-SEM; Philips XL 30 and S-4160) was employed for determining the size and morphology of the catalyst and elements of catalyst were identified by energy-dispersive X-ray (EDX) spectroscopy capability. Powder X-ray diffraction (XRD) analysis of the samples was recorded using by a Philips X-Pert 1710 diffractometer employing Co Kα (λ =1.78897 Å) at a voltage of 40 kV and current of 40 mA. Magnetization measurements were performed by a vibrating magnetometer/alternating gradient force magnetometer (MDKCo, Iran, www.mdk-magnetic.com). Thermogravimetric analysis (TGA) was studied under flowing N2 by a Bahr STA-503 instrument with the temperature at 25 °C to 900 °C.
2.3 General Procedure for Synthesis of 4-aryl-NH-1, 2, 3-triazoles
A round-bottomed flask (10 mL) was charged with benzyl alcohol (1.0 mmol), sodium azide (2.0 mmol), H2O2 (3 mmol) and nitromethane (2.0 mmol) were added in ethanol (4 mL). Afterwards, the mixture was refluxed in the presence of 40 mg Fe3O4@SiO2@TCT-DABCO-SO3H magnetic catalyst. After completion of the reaction, the magnetic catalyst was separated by using an external magnet, finally, the product had to be isolated by column chromatography on silica gel (70-230 Mesh) using n-hexane–ethyl (4:1) acetate as eluent.
3 Results and Discussion
FT-IR of Fe3O4@SiO2@TCT-DABCO (Red curve), Fe3O4@SiO2@TCT (Blue curve) Fe3O4@SiO2@TCT-DABCO-SO3H before reaction (Orange curve) and after reaction (Yellow curve) was depicted in Figure 1. The absorption bands at around 586 cm−1 are associated with vibrating bands for Fe-O groups. The band Si-O-Si stretching vibration appeared at around 1079 cm−1. The absorption bands at 1398 cm−1 in Fe3O4@SiO2@TCT (Blue) is related to band C-N. Also, the peaks at 1558 cm−1, 1614 cm−1 and 1700 cm−1 in Fe3O4@SiO2@TCT-DABCO-SO3H (Orange) are related to cyanuric chloride. The O-H stretch of Fe3O4@SiO2@TCT-DABCO-SO3H (Red), was stronger than that of Fe3O4@SiO2@TCT-DABCO (Red) at around 3386 cm−1 which can be attributed to the OH groups of SO3H. Also, it shows that the Fe3O4@SiO2@TCT-DABCO-SO3H nanoparticles have the same structure after 5 cycles (Yellow curve) and this the structure is stable.
The magnetic possession of the Fe3O4@SiO2@TCT-DABCO-SO3H was studied by The VSM. Magnetization curve of the nanocatalyst (emu/g) as a function of the applied field (Oe) shows from −10000 to 10000 Oe, the magnetic saturation of Fe3O4@SiO2@TCT-DABCO-SO3H were around 39.65 emu g−1. The hysteresis loop of the Fe3O4@SiO2@TCT-DABCO-SO3H nanoparticles shows a superparamagnetic behavior which Fe3O4@SiO2@TCT-DABCO-SO3H nanocatalyst can be easily removed by an external magnet from the reaction mixture (Figure 2).
XRD pattern of Fe3O4@SiO2@TCT-DABCO-SO3H was represented in Figure 3. The seven peaks of 21.03, 35.41, 41.63, 49.99, 63.37, 67.65 and 74.59 corresponds to the crystal faces (111), (220), (311), (400), (422), (511) and (440), (JCPDS card no. 19-0629) which confirms a magnetic cubic structure of Fe3O4. The presence of the sulfated DABCO did not significantly affect the crystalline cubic spinel structure of the Fe3O4 nanoparticles.
The particle size and the morphology of Fe3O4@SiO2@TCT-DABCO-SO3H were investigated using SEM. It was verified that the nanoparticles were synthesized between 40 nm to 50 nm in size. Also, it shows that the Fe3O4@SiO2@TCT-DABCO-SO3H nanoparticles have a spherical morphology and a uniform structure (Figure 4).
The size and the morphology of Fe3O4@SiO2@TCT-DABCO-SO3H were investigated by using TEM that the Fe3O4@SiO2@TCT-DABCO-SO3H nanoparticles were orderly decorated with spherical particles with particles size of about 40 to 50 nm (Figure 5).
To investigate the elemental composition of Fe3O4@SiO2@TCT-DABCO-SO3H, EDX was obtained. The EDX spectrum of nanocatalyst shows the presence of carbon, oxygen, nitrogen, chlorine, sulfur, ferrite and silicon atoms (Figure 6). The amount of Fe was determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES) that was 15 mmol/g.
Quantitative determination of the organic ligand loaded on the surface of magnetic nanocatalyst was carried out by TGA (Figure 7). The TGA diagram of the nanoparticle was performed under N2. Nearly 10 mg of sample was heated at a rate of 20 °C/min within the range of 20 °C–800 °C. The first peak appeared around 200 °C with weight loss of about 5% owing to evaporation of adsorbed moisture. The second and main loss in weight (12%) in the range 200 °C–700 °C, corresponds to the degradation of the covalently bonded ligand loaded on the magnetic nanoparticle. Furthermore, according to the TGA curve, the magnetic catalyst was stable up to 200 °C.
We have studied the optimum condition of reaction. The reaction between benzyl alcohols, nitromethane and sodium azide was applied as a model, and various solvent, different amount of catalyst and oxidant were used to optimize the reaction (Scheme 2).
The use of ethanol and hydrogen peroxide led to a much better result than with conventional organic solvents including DMF, H2O, xylene, toluene, and mesitylene at 78 °C for 4 h. Ethanol has been used as a green solvent in the synthesis of 4-aryl-NH-1, 2, 3-triazoles where it facilitated the solubility of the organic reagent. Also, the different oxidants were investigated such as H2O2, tert-Butyl hydroperoxide (TBHP) and meta-Chloroperoxybenzoic acid (MCPBA) for the oxidation benzyl alcohol to benzaldehyde that the best result is related to H2O2. In the absence of catalyst, no product was found (Table 1, Entry 1) and when we used Fe3O4@SiO2@TCT-DABCO-SO3H as catalyst and ethanol as solvent it led to the formation of 4-aryl-NH-1, 2, 3-triazole in the acceptable yield (Table 1, Entry 2). Also, the amount of catalyst was optimized and we found that the best performance of the catalyst was resulted by 40 mg of the catalyst by providing a maximum of 90% yield (Table 1, Entry 2). Also, this reaction was performed in the presence of FeCl3 and FeCl3/ p-toluenesulfonic acid (TsOH) as a catalyst, and 0% and 55% yields were found, respectively (Table 1, Entry 18 and 19). This reaction was tested without H2O2 that the reaction was not performed. Also, this reaction was tested in the presence of H2O2 and TsOH such that the yield of the reaction was 30-40%. Therefore, para toluene sulfonic acid helped in the oxidation reaction. Finally, the reaction was performed in the presence of H2O2 and Fe3O4@SiO2@TCT-DABCO-SO3H such that the yield of the reaction was 95%.
Also, the sulfonation of the catalyst was compared with Fe3O4@SiO2@TCT-DABCO (Table 2). It was found that the acidic activity of magnetic catalyst due to sulfonation of DABCO on the magnetic surface that contributes to oxidation of benzyl alcohols was improved by using hydrogen peroxide (Table 2, entry 2). Also, the hydrogen bond of sulfonic acid of Fe3O4@SiO2@TCT-DABCO-SO3H with intermediate can affect in the progressive improvement of reaction due to this active hydrogen bond intermediate.
Under optimized conditions 4-aryl-NH-1, 2, 3-triazole derivatives were prepared in excellent yields, via oxidation benzyl alcohols with hydrogen peroxide and next cycloaddition of aryl aldehyde, nitromethane and sodium azide in the presence of a 40 mg of the Fe3O4@SiO2@TCT-DABCO-SO3H at 78 °C.
To explore the scope of the reaction, we investigated this reaction under the optimized condition for various types of benzyl alcohol, sodium azide, H2O2 and nitromethane and the results are listed in Table 3. Notably, click chemistry of benzyl alcohol, (3-nitrophenyl)methanol or p-tolylmethanol with sodium azide and nitromethane provided excellent yields (Table 3 entries 1, 4 and 10). The reaction was carried out with difficulty to produce the product in 35-40% yield when the (4-nitrophenyl)methanol was used (Table 1, entry 6). (4-Methoxyphenyl)methanol or (4-chlorophenyl)methanol reacted with sodium azide and nitromethane provide good yield (Table 3, entry 2 and 5).
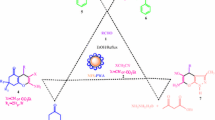
An acceptable mechanism for the three-part reaction of benzyl alcohol, nitromethane and sodium azide in the vicinity of magnetic nanoparticles operating with Fe3O4@SiO2@TCT-DABCO-SO3H as a catalyst in Scheme 3 are shown. According to this mechanism, benzyl alcohol is first converted to benzaldehyde by a proposed catalytic cycle based on the sulfonic acid group.19 In the next step, the benzaldehyde produced by the sulfonic acid group is activated by hydrogen bonding between the sulfonic acid groups and the oxygen atom of the aldehyde group, which increases the property of the electrophilic aldehyde. Next, the nitromethane is dehydrogenized by nitrogen atoms of the catalyst and is reacted with the aldehyde and the nitro vinyl benzene is produced. Then sodium azide is reacted via the [3+ 2] cycloaddition. Finally, the sulfonic acid group of catalyst helps to exit HNO2 and form a triazole.20
The reusability of the Fe3O4@SiO2@TCT-DABCO-SO3H catalyst was investigated using the reaction of benzyl alcohol, nitromethane and sodium azide as a model reaction. The recyclability of catalyst is observed in Figure 8, which can demonstrate the stability of this catalyst. Also, it is observed that the Fe3O4@SiO2@TCT-DABCO-SO3H catalyst can be recovered and reused at least five times without noticeable loss of catalytic activity.
The results obtained for the synthesis of 4-aryl-NH-1, 2, 3-triazoles under the optimized conditions were compared with previously reported catalysts in the literature. It can be seen in Table 4 that the present catalyst showed good catalytic activity. Noticeably, this new catalyst is comparable in terms of eco-friendly, reusability, commercially available materials, easy preparation, and facile separation. Also, this method is performed in a green solvent and short time.
4 Conclusions
In this study, Fe3O4@SiO2@TCT-DABCO-SO3H as the novel and heterogeneous catalyst was synthesized and characterized for the multicomponent reaction of various type of benzaldehyde, sodium azide, and nitromethane also the three-part reaction of benzyl alcohol, nitromethane, and sodium azide was used based on an oxidative step for the synthesis of these compounds. This method was used for the development of the “one-pot” synthesis of 4-aryl-NH-1, 2, 3-triazole in good yield which is economical and the separation of the intermediates is avoided. Also, recovery of the magnetic catalyst was performed five times without a significant loss of high catalytic performance.
Reference
(a) Chabre Y M and Roy R 2008 Recent trends in glycodendrimer syntheses and applications Curr. Top. Med. Chem. 8 1237; (b) Colombo M and Peretto I 2008 Chemistry strategies in early drug discovery: an overview of recent trends Drug. Discov. Today 13 677; (c) Hanselmann R, Job G E, Johnson G and Lou R L J G 2010 Synthesis of an antibacterial compound containing a 1, 4-substituted 1 H-1, 2, 3-triazole: A scalable alternative to the “click” reaction Org. Process Res. Dev. 14 152; (d) Moumne R, Larue V, Seijo B, Lecourt T, Micouin L and Tisne C 2010 Org. Biomol. Chem. 8 1154
He X P, Xie J, Tang Y, Li J and Chen G R 2012 CuAAC click chemistry accelerates the discovery of novel chemical scaffolds as promising protein tyrosine phosphatases inhibitors Curr. Med. Chem. 19 2399
Hu L, Mück-Lichtenfeld C, Wang T, He G, Gao M and Zhao J 2016 Reaction between Azidyl Radicals and Alkynes: A Straightforward Approach to NH-1, 2, 3-Triazoles Chem. Eur. J. 22 911
(a) Jin T, Kamijo S and Yamamoto Y 2004 Copper-Catalyzed Synthesis of N-Unsubstituted 1, 2, 3-Triazoles from Nonactivated Terminal Alkynes Eur. J. Org. Chem. 18 3789; (b) Kalisiak J, Sharpless K B and Fokin V V 2008 Efficient synthesis of 2-substituted-1, 2, 3-triazoles Org. Lett. 10 3171; (c) Cohrt A E, Jensen J F and Nielsen T E 2010 Traceless Azido Linker for the Solid-Phase Synthesis of N H-1, 2, 3-Triazoles via Cu-Catalyzed Azide–Alkyne Cycloaddition Reactions Org. Lett. 12 5414
Barluenga J, Valdés C, Beltrán G, Escribano M and Aznar F 2006 Developments in Pd Catalysis: Synthesis of 1H-1, 2, 3-Triazoles from Sodium Azide and Alkenyl Bromides Angew. Chem. Int. Edit. 45 6893
Quan X J, Ren Z H, Wang Y Y and Guan Z H 2014 p-Toluenesulfonic acid mediated 1, 3-dipolar cycloaddition of nitroolefins with NaN3 for synthesis of 4-aryl-NH-1, 2, 3-triazoles Org. Lett. 16 5728
Zhang W, Kuang C and Yang Q 2010 Palladium-catalyzed one-pot synthesis of 4-aryl-1H-1, 2, 3-triazoles from anti-3-aryl-2, 3-dibromopropanoic acids and sodium azide Synthesis 2010 283
(a) Bhuyan P, Bhorali P, Islam I, Bhuyan A J and Saikia L 2018 Magnetically recoverable copper ferrite catalyzed cascade synthesis of 4-Aryl-1H-1, 2, 3-triazoles under microwave irradiation Tetrahedron Lett. 59 1587; (b) Mirzaei-Mosbat M, Ghorbani-Vaghei R and Sarmast N 2019 One-pot Synthesis of 4-Aryl-NH-1,2,3-triazoles in Presence of Fe3O4@SiO2@Propyl-HMTA as a New Basic Catalyst ChemistrySelect 4 1731; (c) Wu L, Wang X, Chen Y, Huang Q, Lin Q and Wu M 2016 4-Aryl-NH-1, 2, 3-Triazoles via Multicomponent Reaction of Aldehydes, Nitroalkanes, and Sodium Azide Synlett 27 437
Johnson J A, Baskin J M, Bertozzi C R, Koberstein J T and Turro N J 2008 Copper-free click chemistry for the in situ crosslinking of photodegradable star polymers Chem. Commun. 26 3064
Gierlich J, Burley G A, Gramlich P M, Hammond D M and Carell T 2006 Click chemistry as a reliable method for the high-density postsynthetic functionalization of alkyne-modified DNA Org. Lett. 8 3639
Polshettiwar V, Baruwati B and Varma R S 2009 Magnetic nanoparticle-supported glutathione: A conceptually sustainable organocatalyst Chem. Commun. 14 1837
Baig RN and Varma R S 2013 Copper on chitosan: a recyclable heterogeneous catalyst for azide–alkyne cycloaddition reactions in water Green Chem. 15 1839
Polshettiwar V, Baruwati B and Varma R S 2009 Nanoparticle-supported and magnetically recoverable nickel catalyst: a robust and economic hydrogenation and transfer hydrogenation protocol Green Chem. 11 127
Polshettiwar V and Varma R S 2010 Nano-organocatalyst: magnetically retrievable ferrite-anchored glutathione for microwave- assisted Paal–Knorr reaction, aza-Michael addition, and pyrazole synthesis Tetrahedron 66 1091
Dyson P J and Jessop P G 2016 Solvent effects in catalysis: rational improvements of catalysts via manipulation of solvent interactions Catal. Sci. Technol. 6 3302
Johnson J A, Baskin J M, Bertozzi C R, Koberstein J T and Turro N J 2008 Copper-free click chemistry for the in situ crosslinking of photodegradable star polymers Chem. Commun. 26 3064
Capello C, Fischer U and Hungerbühler K 2007 what is a green solvent? A comprehensive framework for the environmental assessment of solvents Green Chem. 9 927
Yazdani E, Azizi K and Nakisa A 2015 Boric Acid-Functionalized Fe3O4@SiO2 as a Novel Superparamagnetically Recoverable Nano Catalyst for Mukaiyama-Aldol Reaction J. Org. Chem. 1 27
Jain S L and Sain B 2006 Perfluorinated resinsulphonic acid (Nafion-H1) catalyzed highly efficient oxidations of organic compounds with hydrogen peroxide Appl. Catal. A 301 259
(a) Liu L, Ai Y, Li D, Qi L, Zhou J, Tang Z and Sun H B 2017 Recyclable Acid–Base Bifunctional Core–Shell–Shell Nanosphere Catalyzed Synthesis of 5-Aryl-1H-1, 2, 3-triazoles through the “One-Pot” Cyclization of Aldehydes, Nitromethane, and Sodium Azide Chem. Cat. Chem. 9 3131; (b) Paris E, Bigi F, Cauzzi D, Maggi R and Maestri G 2018 Oxidative dimerization of anilines with heterogeneous sulfonic acid catalysts Green Chem. 20 382; (c) Jain S L and Sain B 2006 Perfluorinated resinsulphonic acid (Nafion-H®) catalyzed highly efficient oxidations of organic compounds with hydrogen peroxide Appl. Catal. A 301 259
Wua L, Wanga X, Chena Y, Huanga Q, Lin Q and Wua M 2016 4-Aryl-NH-1,2,3-Triazoles via Multicomponent Reaction of Aldehydes, Nitroalkanes, and Sodium Azide Synlett 27 437
Liu L, Ai Y, Li D, Qi L, Zhou J, Tang Z, Shao Z, Liang Q and Sun H-B 2017 Recyclable Acid-Base Bifunctional Core-Shell-Shell Nanosphere Catalyzed Synthesis of 5-Aryl-NH-1,2,3-triazoles via “One-Pot” Cyclization of Aldehyde, Nitromethane and NaN3 Chem. Cat. Chem. 3131
Hu Q, Liu Y, Deng X, Li Y and Chen Y 2016 Aluminium(III) Chloride-Catalyzed Three-Component Condensation of Aromatic Aldehydes, Nitroalkanes and Sodium Azide for the Synthesis of 4-Aryl-NH-1,2,3-triazoles Adv. Synth. Catal. 358 1689
Bhuyan P, Bhorali P, Islam I, Bhuyan A J and Saikia L 2018 Magnetically recoverable copperferrite catalyzed cascade synthesis of 4-Aryl-NH-1,2,3-triazolesunder microwave irradiation Tetrahedron Lett. 59 1587
Li D, Liu L, Tian Y, Ai Y, Tang Z, Sun H-B and Zhang G 2017 a flow strategy for the rapid, safe and scalable synthesis of N-H 1,2,3-triazoles via acetic acid mediated cycloaddition between nitroalkene and NaN3 Tetrahedron 73 3959
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Tarbiat Modares University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
JADIDI NEJAD, M., PAZOKI, F., BAGHERI, S. et al. 1, 4-Diazabicyclo[2.2.2]octane-sulfonic acid immobilized on magnetic Fe3O4@SiO2 nanoparticles: a novel and recyclable catalyst for the one-pot synthesis of 4-aryl-NH-1, 2, 3-triazoles. J Chem Sci 132, 62 (2020). https://doi.org/10.1007/s12039-020-01761-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-01761-w